 © VALERO DOVAL/GETTY IMAGES
© VALERO DOVAL/GETTY IMAGES
On a closed-circuit television I watch Marie settle into her room, unpacking her toiletries in the bathroom and arranging her clothes for the next day. Her digs at the University of Chicago sleep lab look like an ordinary hotel room, with a bed, TV, desk, nightstand. Ordinary, except for the camera keeping watch from across the bed and the small metal door in the wall next to the headboard. The door, about one foot square, is used when researchers want to sample the study participants’ blood during the night without disturbing them; an IV line passes from the person’s arm through the door and into the master control room where I’m watching Marie on the screen.
She’s come to the lab on a weekday evening to be screened for possible inclusion in a study on insomnia. Marie says her sleep problems started almost 20 years...
After a few years, Marie (not her real name—she asked to remain anonymous for privacy) stopped working. Most nights she’ll sleep for a short stretch—maybe a few hours—then wake up and lie awake for hours as pain in her neck consumes her and makes her uneasy and restless. “I’ve seen psychologists, physical therapists, doctors. I’ve been prescribed medications for depression. But it didn’t work,” she says. “Every single day it’s a struggle . . . I feel like when Job was attacked by the devil. Someone is trying to take my vitality away.”
A lack of sleep can do just that, sometimes with fatal consequences. Experiments have shown that keeping animals awake for days on end can kill them, for example, and in 2012, news outlets reported that a soccer fan had died after 11 sleepless nights spent watching the sport on television. “I think we forget that sleep is a basic physiological requirement,” says Carol Everson, who studies the effects of sleep deprivation at the Medical College of Wisconsin.
Death is obviously an extreme consequence resulting from extreme sleep deprivation, but many of the millions of people who suffer from less-intense sleep problems do suffer myriad health burdens. In addition to emotional distress and cognitive impairments, these can include high blood pressure, obesity, and metabolic syndrome. And recent research has suggested even mild sleep loss, the kind people often subject themselves to during the work week by watching late-night TV until midnight then rising before dawn, may lead to metabolic, cardiovascular, cognitive, and neurologic dysfunction.
“In the studies we’ve done, almost every variable we measured was affected. There’s not a system in the body that’s not affected by sleep,” says University of Chicago sleep researcher Eve Van Cauter. “We’re not wired for sleep deprivation. Every time we sleep-deprive ourselves, things go wrong.”
A brain without sleep
 © ISTOCK.COM/MAJORDESIGNSSleep deprivation experiments go back more than a century, as researchers have sought to understand the function of sleep. A common refrain among sleep scientists until about two decades ago was that sleep was performed by the brain in the interest of the brain. That was an incomplete insight, but it was not wrong. Numerous studies have hinted at the function of sleep by confirming that neurological function and cognition are messed up during sleep loss, with reaction time, mood, and judgment suffering from being awake for too long.
© ISTOCK.COM/MAJORDESIGNSSleep deprivation experiments go back more than a century, as researchers have sought to understand the function of sleep. A common refrain among sleep scientists until about two decades ago was that sleep was performed by the brain in the interest of the brain. That was an incomplete insight, but it was not wrong. Numerous studies have hinted at the function of sleep by confirming that neurological function and cognition are messed up during sleep loss, with reaction time, mood, and judgment suffering from being awake for too long.
Undergirding these behavioral changes are sleep deprivation’s effects on basic brain function. Electroencephalogram recordings show disruptions in normal brain-activity patterns—abnormalities that are thought to result from altered neural activity in brain regions such as the cortex, basal forebrain, hippocampus, and striatum. The latter has been found to downregulate dopamine receptors, which may help maintain arousal. Hippocampal neurons, meanwhile, show a reduction in long-term potentiation, a form of synaptic plasticity that normally reinforces connections between brain cells and underlies memory formation. And the sleep-deprived basal forebrain—one of the brain’s main wakefulness centers—experiences an increased release of nitric oxide leading to a buildup of adenosine, a nucleoside that can also affect neural function.
Decades-old studies found that adenosine functioned as a sedative when given to animals. For years, Bob McCarley of Harvard Medical School has probed the role of adenosine in sleep, and, in particular, sleepiness. In 1997, he and his colleagues found that when they kept cats awake by playing with them, extracellular adenosine increased in the basal forebrain as the sleepy felines stayed up longer and slowly returned to normal levels when they were later allowed to sleep.1 McCarley’s team also found that administering adenosine to the basal forebrain put animals to sleep. (It should come as no surprise, then, that caffeine—which blocks adenosine’s receptors—keeps us awake.)
When people reported sleeping less than six hours per night they were almost always overweight or obese.—John Pollock,
Duquesne University
Teaming up with Radhika Basheer and others, McCarley discovered that, as adenosine levels rise during sleep deprivation, so do the concentrations of adenosine receptors, magnifying the molecule’s sleep-inducing effect.2 “The brain has cleverly designed a two-stage defense against the consequences of sleep loss,” McCarley says.
Adenosine may underlie some of the cognitive deficits that result from sleep loss. Harvard Medical School’s Robert Strecker, McCarley, and colleagues found that infusing adenosine into rats’ basal forebrain impaired their performance on an attention test, similar to what is seen in sleep-deprived humans and animals.3 But adenosine levels are by no means the be-all and end-all of sleep deprivation’s effects on the brain or the body.
Systemic effects
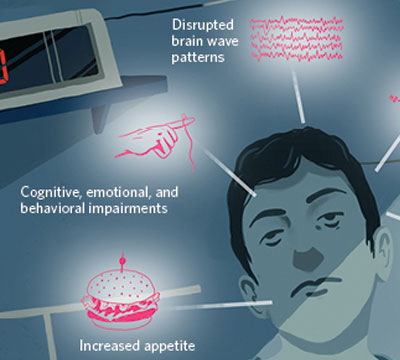 UNDER THE COVER OF DARKNESS: Strange things happen at night, especially when we’re not sleeping. Results from observational studies of people who don’t sleep much and from experiments on human volunteers have revealed that the consequences of sleep deprivation are far-reaching, from molecules and cells to organs and behavior.
UNDER THE COVER OF DARKNESS: Strange things happen at night, especially when we’re not sleeping. Results from observational studies of people who don’t sleep much and from experiments on human volunteers have revealed that the consequences of sleep deprivation are far-reaching, from molecules and cells to organs and behavior.
See full infographic: WEB | PDF© EROS DERVISHIEven the earliest sleep-restriction experiments on people revealed untoward consequences for the body, not just the brain. In 1896, George Thomas White Patrick and J. Allen Gilbert of the University of Iowa kept three of their university colleagues awake for more than 88 hours and recorded the effects: visual hallucinations, decreased grip and pull strength, and slowed reaction time. Curiously, the participants also gained 0.5 to 1.5 kg by the end of the sleep deprivation period, and lost the extra kilos as soon as they resumed normal sleep.4 “The steady increase in the subject’s weight during the experiment and the sudden decrease in weight after sleep are noteworthy, and apparently not to be accounted for by accidental circumstances,” Patrick and Gilbert wrote of their first volunteer.
A century later, real-world observations found a similar link. “The epidemiologic studies . . . kept showing over and over again that when people reported sleeping less than six hours per night they were almost always overweight or obese,” says David Dinges, who studies sleep loss at the University of Pennsylvania Perelman School of Medicine. “And it wasn’t just obesity.” People who slept very little (often less than six hours per night) were more likely to be diabetic or suffer a heart attack as well. “This led to an increasing medical understanding that sleep may be playing a role in things we never thought possible,” says Dinges. (Interestingly, the data also revealed adverse effects of sleeping for too long, say, more than eight or nine hours every night, including an increased risk of early death.)
These were only observational studies, however. To see if there could be any causal relationship between sleep patterns and the metabolic outcomes, researchers had to bring subjects into the lab. So in 1999, Van Cauter and her colleagues had 11 men sleep in the lab at the University of Chicago. For three nights, the volunteers spent eight hours in bed, then for six nights they were allowed only four hours in bed (accruing what Van Cauter calls a sleep debt), and then for six nights the men could sleep for up to 12 hours (sleep recovery). During sleep debt and recovery, researchers gave the participants a glucose tolerance test and found striking differences. While sleep deprived, the men’s glucose metabolism resembled a prediabetic state.5 “We knew it would be affected,” says Van Cauter. “The big surprise was the effect size was much greater than we thought.”
Subsequent studies also found insulin resistance increased during bouts of sleep restriction. In 2012, Van Cauter’s team took fat biopsies from seven adults who spent four-and-a-half hours in bed for four nights, and the researchers found impairments in insulin signaling in their adipocytes.6 The participants’ fat cells “were no longer responding to one of the major modulators of their function. That’s what impressed me,” Van Cauter says. “You could almost see that the cell was sleep deprived.”
It’s possible these immediate metabolic changes could explain the longer-term health impacts seen among people who don’t get much shut-eye. Overeating is another possible explanation. In some research using human subjects, levels of the appetite-suppressing hormone leptin have gone down upon sleep restriction, while levels of ghrelin, a hunger promoter, have gone up, spurring a greater desire for food, especially calorie-dense snacks. One recent study showed that sleep-restricted people will add 300 calories to their daily diet.7 “In our brain we have a system that links sleeping and fasting or being awake and eating. So when you sleep-deprive yourself, the system is thinking there must be a lack of food and therefore you begin to forage at the refrigerator,” says Van Cauter.
Echoing Van Cauter’s results, Basheer has found evidence that sleep deprivation sends the brain into a catabolic, or energy-consuming, state.8 It degrades the energy molecule adenosine triphosphate (ATP) to produce adenosine monophosphate and results in the activation of AMP kinase, an enzyme that boosts fatty acid synthesis and glucose utilization. “The system sends a message there is a need for more energy,” Basheer says. Whether this is indeed the mechanism underlying late-night pig-outs is still speculative.
Gene-expression analyses also highlight the metabolic mayhem wrought by sleep restriction. In 2013, the University of Surrey’s Simon Archer, Derk-Jan Dijk, and colleagues monitored volunteers who slept about six hours a night for one week and eight hours each night for another. At the end of each week, they prevented the participants from sleeping for a continuous 40 hours and sampled their blood over a couple of days to look for genes that were differentially expressed. The expression of genes associated with metabolism was all out of whack after a week of insufficient sleep. But that wasn’t all. Transcriptome analysis showed that many genes linked with immune and inflammatory processes and with gene regulation were also up- or downregulated after the week of sleep deprivation.9
These and other studies make it clear that insufficient sleep can have profound effects on physiology. Yet most experiments have spanned fewer than two weeks. One open question is whether chronic sleep loss can produce long-term changes. Preliminary research suggests that it can.
Long-term consequences
One of the assumptions in the sleep field has been that the effects of sleep deprivation are temporary, and that after people resume a normal sleep schedule, their cognitive performance, brain chemistry, and physiology go back to baseline. In animals, researchers have found evidence that the effects of sleep deprivation can last for months. For 10 weeks, Carol Everson of the Medical College of Wisconsin subjected rats to repeated cycles of 10 days of disrupted and limited sleep followed by two days without disturbance. During the first half of the experiment, the animals seemed perfectly healthy. But after about five weeks, problems emerged. “All of a sudden their food intake took off progressively and they weren’t gaining weight,” Everson says.
In addition, the rodents’ fur lost its luster, their adipocytes were smaller and of a different phenotype than control animals’, and their small intestines were longer by 30 percent.10 Longer intestines typically correlate with increased surface area for nutrient and water absorption, says Everson. It’s not clear yet why the sleep-deprived rodents weren’t gaining weight, as sleep-deprived humans do. This apparent discrepancy between the species “will become resolved when we better understand the inherent properties of the metabolic challenge posed by sleep loss and the context in which it occurs,” Everson says, noting that individual differences in physical constitution or varying sleep-restriction experimental paradigms could contribute.
To test for a possible lifetime burden from the experience, Everson put another set of animals through the same 10-week regimen, then let them go back to their normal sleep behavior for four months. While some of the changes returned to baseline, metabolic abnormalities persisted—the rats were still eating more food, yet their weight was no different than control animals’.11 And “then there were emergent changes, things we had not seen before,” says Everson. Leptin levels increased, for instance. “It’s a sign that there’s an energy deficit, and leptin insensitivity, which is a component of obesity.”
Within the brain, scientists have glimpsed signs of physical damage from sleep loss, and the time line for recovery, if any occurs, is unknown. Chiara Cirelli’s team at the University of Wisconsin–Madison School of Medicine has found structural changes in the cortical neurons of mice when the animals are kept awake for long periods of time. Specifically, Cirelli and her colleagues saw signs of mitochondrial activation—which makes sense, as “neurons need more energy to stay awake,” she says—as well as unexpected changes, such as increased lysosomal activation and undigested cellular debris, signs of cellular aging that are unusual to see in the neurons of young, healthy mice.12
“The number [of debris granules] was small, but it’s worrisome because it’s only four to five days” of sleep deprivation,” says Cirelli. And after 36 hours of sleep recovery—an amount of time in which she expected normalcy to resume—those changes remained.
Sigrid Veasey’s team at Penn has also noticed changes in the brains of mice after several days of sleep loss—in this case, locus ceruleus neurons (LCns), which are active during waking, were found to degenerate. A short bout of sleep deprivation—three hours kept awake—sparked a protective mitochondrial response in the cells, but disrupting the animals’ sleep for a few days was damaging. “While it is difficult to discern whether the loss of LCns and injury of this magnitude are sufficient to result in cognitive impairments, we propose that repeated occurrences of [extended sleep deprivation] (as is commonly observed in night-shift workers) could result in a cumulative loss of LCns . . .” the authors wrote in their 2014 paper.13
Veasey’s results “are bizarre and incredible,” says Penn’s Dinges, who adds that the study raises important questions about whether there are permanent effects of sleep loss. “That has me worried about human studies, because if I can’t reverse the sleep-deprivation effects, I must stop immediately.” Dinges is now testing whether sleep loss can have long-lasting effects in humans by limiting volunteers to four hours in bed for five nights, then giving them 2, 4, 6, 8, or 10 hours of recovery time in bed, and then testing their cognitive performance during another bout of sleep restriction.
Shift workers, night owls, and insomniacs like Marie could also lend valuable insight into persistent changes from sleep restriction, serving as natural experiments on how the human body reacts to losing out on a basic life need for chronic periods. She ended up qualifying for the University of Chicago study, which is examining whether insomniacs have hyperactivity in their muscle sympathetic nerves. While numerous labs want to gather experimental data to probe the epidemiological data generated by such brief sleepers, Marie says she’s most interested in fixing her condition. Whenever she reads about advances in sleep research in the news, it’s about the consequences of insomnia, she says, “but not a huge breakthrough” in therapies to treat the effects of sleep deprivation. (See “Desperately Seeking Shut-Eye” here.) With so much of our physiology affected when we lose out on sleep, an effective therapeutic—other than sleep itself—is difficult to imagine.
“People like to define a clear pathway of action [for health conditions],” says Van Cauter. “With sleep deprivation, everything you measure is affected and interacts synergistically to produce the effect.”
 LASTING EFFECTS OF SLEEP LOSS: The short-term consequences of sleep loss are numerous, but whether they leave a lasting scar is unknown. So Carol Everson and Aniko Szabo of the Medical College of Wisconsin subjected rats to a 10-month regime of sleep restriction and then allowed the animals to sleep as they pleased for several months. Compared to control animals, the sleep-deprived rats suffered a variety of physical effects, with some problems persisting even after the recovery period (PLOS ONE, doi:10.1371/journal.pone.0022987, 2011). In another study by Everson and colleagues, bone and bone marrow abnormalities persisted throughout sleep restriction and recovery periods (Exp Biol Med, 237:1101-09, 2012).© EROS DERVISHI
LASTING EFFECTS OF SLEEP LOSS: The short-term consequences of sleep loss are numerous, but whether they leave a lasting scar is unknown. So Carol Everson and Aniko Szabo of the Medical College of Wisconsin subjected rats to a 10-month regime of sleep restriction and then allowed the animals to sleep as they pleased for several months. Compared to control animals, the sleep-deprived rats suffered a variety of physical effects, with some problems persisting even after the recovery period (PLOS ONE, doi:10.1371/journal.pone.0022987, 2011). In another study by Everson and colleagues, bone and bone marrow abnormalities persisted throughout sleep restriction and recovery periods (Exp Biol Med, 237:1101-09, 2012).© EROS DERVISHI
HOW DO YOU SLEEP?
But over many years of giving his subjects this so-called psychomotor vigilance test (PVT), Dinges began to notice that averaging their performance masked variability between them. “Some people were deteriorating at a much faster rate than others,” he says. Nothing predicted it: age, gender, education. “And the longer you went with inadequate sleep, the larger the individual differences got.” To better understand this variation, Dinges recruited 21 volunteers to come to the lab and stay awake for 36 hours, periodically taking the PVT during the deprivation. Then, at two-week intervals, the same subjects came back to repeat the experiment two more times (Sleep, 27:423-33, 2004). “The day came when we repeated the exposure, and lo and behold, we got the same response,” Dinges says. “If you were vulnerable at one time when we tested you, you’re vulnerable again.” It turns out that people fall into one of three categories, which Dinges termed Type 1 (resilient), Type 2 (intermediate), and Type 3 (vulnerable). Interestingly, Type 1 subjects are affected by the experiment—they get sleepy, their eyelids droop, and they struggle to concentrate—but their cognitive performance stays strong, compared with that of more-vulnerable subjects. Nearly a decade later, an independent group at Penn conducted a similar experiment on identical and fraternal twins, finding that resiliency and vulnerability to sleep loss are highly heritable (Sleep, 35:1223-33, 2012). And last year, Penn’s Namni Goel, Dinges’s collaborator, showed that it isn’t just performance on the PVT that varies predictably between the sleep deprived; stable differences in eating behaviors and weight gain also appear between individuals subjected to sleep loss (Scientific Reports, 5:14920, 2015). Dinges and others have looked for genes related to vulnerability phenotype, but they’ve yet to identify any that can explain the different responses to sleep deprivation. Dinges is also keen on finding a biomarker that can predict which type a person is. Such a test could be useful to, say, the military or to transportation agencies, jobs in which people may be operating under poor sleep conditions, he says. “We’d really like to know, how can one person tolerate this so much better than another?” |
References
- T. Porkka-Heiskanen et al., “Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness,” Science, 276:1265-68, 1997.
- R. Basheer et al., “Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain,” Neuroreport, 18:1895-99, 2007.
- M.A. Christie et al., “Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task,” Sleep, 31:1393-98, 2008.
- G.T.W. Patrick, J.A. Gilbert, “Studies from the psychological laboratory of the University of Iowa: On the effects of loss of sleep,” Psychol Rev, 3:469-83, 1896.
- K. Spiegel et al., “Impact of sleep debt on metabolic and endocrine function,” Lancet, 354:1435-39, 1999.
- J.L. Broussard et al., “Impaired insulin signaling in human adipocytes after experimental sleep restriction: A randomized, crossover study,” Ann Intern Med, 157:549-57, 2012.
- J.L. Broussard et al., “Elevated ghrelin predicts food intake during experimental sleep restriction,” Obesity, 24:132-38, 2016.
- M. Dworak et al., “Sleep and brain energy levels: ATP changes during sleep,” J Neurosci, 30:9007-16, 2010.
- C.S. Möller-Levet et al., “Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome,” PNAS, 110:E1132-41, 2013.
- C.A. Everson, A. Szabo, “Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes,” Am J Physiol Regul Integr Comp Physiol, 297:R1430-40, 2009.
- C.A. Everson, A. Szabo, “Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats,” PLOS ONE, 6:e22987, 2011.
- L. de Vivo et al., “Loss of sleep affects the ultrastructure of pyramidal neurons in the adolescent mouse frontal cortex,” Sleep, pii:sp-00267-15, 2015.
- J. Zhang et al., “Extended wakefulness: Compromised metabolomics in and degeneration of locus ceruleus neurons,” J Neurosci, 34:4418-31, 2014.
Interested in reading more?





