Login
Subscriberegulation

FDA Head Leaving Post
Kerry Grens | Feb 6, 2015 | 2 min read
US Food and Drug Administration commissioner Margaret Hamburg is stepping down after six years on the job.

Lazarus Drugs
Kate Yandell | Feb 1, 2015 | 8 min read
While some drugs sail through development, others suffer setbacks, including FDA rejections, before reaching the market.

IOM Urges Data Sharing
Kerry Grens | Jan 15, 2015 | 2 min read
The Institute of Medicine says results from human clinical trials ought to be made available to independent researchers within 18 months.

The Year in Pathogens
Molly Sharlach | Dec 28, 2014 | 4 min read
Ebola, MERS, and enterovirus D68; polio eradication efforts; new regulations on potentially dangerous research

Science Setbacks: 2014
Molly Sharlach | Dec 24, 2014 | 3 min read
This year in life science was marked by paltry federal funding increases, revelations of sequence contamination, and onerous regulations.

Europe Softens on GM Crops
Jef Akst | Dec 9, 2014 | 2 min read
A new agreement in the European Union allows genetically engineered crops to be approved without member-state votes, likely allowing several GMO foods to enter the market.

New NIH IRB Guidelines Proposed
Jef Akst | Dec 8, 2014 | 1 min read
A draft policy from the US National Institutes of Health suggests that clinical studies performed at multiple sites should be reviewed by a single institutional review board.

Dangerous Research Regs Released
Jef Akst | Sep 25, 2014 | 2 min read
The US government releases its policy on so-called dual-use research involving dangerous pathogens that could be used for biological terrorist attacks.

That Loving Feeling
Megan Scudellari | Jul 1, 2014 | 9 min read
There are no FDA-approved drugs to treat low sexual desire in women, but not for lack of trying.

Sex and Drugs
Kerry Grens | Jul 1, 2014 | 3 min read
Did 20th-century pharmaceutical and technological advances shape modern sexual behaviors?

FDA Issues Nanotechnology Guidance
Tracy Vence | Jun 26, 2014 | 1 min read
Four new documents from the US Food and Drug Administration provide industry with guidelines on the use of nanotechnology in products regulated by the agency.

Week in Review: June 16–20
Tracy Vence | Jun 20, 2014 | 2 min read
Early Neanderthal evolution; developing antivirals to combat polio; the mouth and skin microbiomes; insect-inspired, flight-stabilizing sensors

Updated Review: Tamiflu Is a Bust
Kerry Grens | Apr 10, 2014 | 2 min read
After finally getting their hands on full clinical study reports, independent reviewers say the antiviral drug is ineffective.
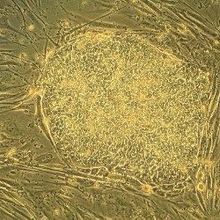
Stem Cell Lines Not Fit for Clinic
Kerry Grens | Feb 6, 2014 | 3 min read
Most stem cell lines registered with the NIH don’t comply with the FDA’s guidelines for human use, according to a new report.

Inconsistent Evidence
Jef Akst | Jan 22, 2014 | 1 min read
More than a third of US drug approvals are based on a single large clinical trial, while others require more in-depth study, according to a new report.

GeneLink Settles
Abby Olena, PhD | Jan 14, 2014 | 2 min read
Another personal genomics company faces government pressure—this time from the Federal Trade Commission.

23andMe Steps Back
Abby Olena, PhD | Dec 6, 2013 | 2 min read
The company announces that it will stop offering health interpretations of personal genetic data.

Capsule Reviews
Bob Grant | Nov 1, 2013 | 4 min read
Tracks and Shadows, The Gap, The Cure in the Code, and An Appetite for Wonder

EU Considers Curbing Antibiotic Use
Chris Palmer | Jul 30, 2013 | 2 min read
The European Medicines Agency has recommended that farmers stop using the antibiotic colistin prophylactically, citing human health concerns.

Opinion: Racing Toward Invention
George Lewis | Jul 23, 2013 | 3 min read
A newly instated patent law discriminates against academics and small biotechs.