 SIFTING STEM CELLS: Gold nanoparticles carrying antibodies to stem cell surface markers bind to the cells and enhance spectral signals detected by surface-enhanced Raman spectroscopy (SERS). J. HAN ET AL., BIOMATERIALS, doi:10.1016/j.biomaterials.2016.07.033, 2016.
SIFTING STEM CELLS: Gold nanoparticles carrying antibodies to stem cell surface markers bind to the cells and enhance spectral signals detected by surface-enhanced Raman spectroscopy (SERS). J. HAN ET AL., BIOMATERIALS, doi:10.1016/j.biomaterials.2016.07.033, 2016.
When a laser beam shines on a single cell, chemicals within the cell can absorb, reflect, or scatter the light waves. The scattered light generates a unique signature at various wavelengths that can be used to identify specific molecules, depending on the molecule type—protein, sugar, or nucleic acid—and the chemical bonds present within its structure. For decades, researchers have used these signatures, known as Raman spectra, as chemical fingerprints to characterize cells.
Unlike flow cytometry, microfluidics, and other cell sorting methods, techniques that rely on Raman spectroscopy do not require labels, and they can be more sensitive and specific than flow cytometry for many applications. Variant versions such as surface-enhanced Raman spectroscopy (SERS)—where chemicals are...
With quickly improving tools and analytical methods, Raman spectroscopy is gaining popularity among life scientists. “Raman spectroscopy instrumentation has been developing very rapidly in the last five years, with very much improved detectors and light sources reducing the cost, but retaining performance of these systems,” says Duncan Graham, a professor of chemistry at the University of Strathclyde in Glasgow. Faster cameras allow spectra to be captured in microseconds rather than seconds, and better software and statistical tools make it possible to analyze large data sets produced by sorting and analyzing cells, he adds.
Here, The Scientist takes a tour of four strategies that deploy Raman spectroscopy to detect and study cells.
TESTING STEM CELL THERAPIES
Investigators: Chunhui Xu, associate professor of pediatrics, and Ximei Qian, assistant professor of biomedical engineering, Emory University
Project: Removing residual cancer-causing stem cells from preparations derived from pluripotent human stem cells.
Problem: Human pluripotent stem cells (hPSCs) are a promising route to stem cell–based therapies for a wide range of diseases. Cells derived from hPSCs often contain residual undifferentiated cells, however, and these contaminating remnants can form tumors when transplanted into animals. Flow cytometry and other assays can only detect these cells if they form 0.1 percent or more of a population, but a mouse study of tumor formation by undifferentiated cells estimated that concentrations of even 1 in 105 cells (0.001 percent) have the potential to form teratomas (Cell Cycle, doi:10.4161/cc.8.16.9353, 2009). Tumor formation after transplantation of hPSCs into animal models is the “gold standard” test for purity and safety, says Qian, but this process takes about three months. It is also expensive and nonquantitative, making it a poor choice for a clinical assay.
Solution: Xu, Qian, and colleagues wanted to use SERS to detect residual stem cells, as they had done with circulating tumor cells in 2011. The team coated gold nanoparticles with antibodies to two stem cell surface markers known as SSEA-5 and TRA-1-60, and then covered the exposed nanoparticle surfaces with polyethylene glycol (PEG) to reduce nonspecific binding. They tested the nanoparticles on samples of hPSCs mixed with known numbers of fibroblast cells to confirm that the SERS signal was proportional to the number of stem cells present. The assay could detect one stem cell in a background of one million fibroblasts, 2,000- to 15,000-fold more sensitive than flow cytometry assays. The technique worked equally well in a culture of hPSC-derived heart muscle cells. “The high sensitivity and specificity is the key advantage of this assay over other methods,” Xu says.
The assay proved to be as sensitive as the 2011 study of tumor cells, she adds. Optimizing the technique for different cell types requires finding the right ligand density per nanoparticle—in this case, 25 antibodies per particle for the SSEA-5 marker (Biomaterials, doi:10.1016/j.biomaterials.2016.07.033, 2016).
SCANNING FOR SPECIFICITY
Investigators: Iwan Schie, postdoctoral researcher, and Christoph Krafft, research group leader, Leibniz Institute of Photonic Technology, Germany
Project: Improving the specificity of single-cell classification by gathering mean Raman spectra
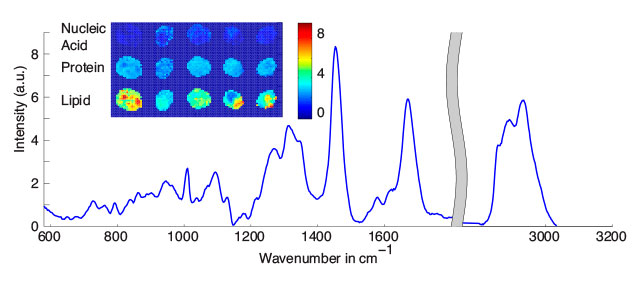 PATCHY CELLS: Cell images (inset) reconstructed using mean Raman spectra acquired from the entire cell (graph) show that macromolecules are unevenly distributed throughout cells.COURTESY IWAN SCHIE
PATCHY CELLS: Cell images (inset) reconstructed using mean Raman spectra acquired from the entire cell (graph) show that macromolecules are unevenly distributed throughout cells.COURTESY IWAN SCHIE
Problem: Most studies rely on a single Raman spectrum reading of a cell. But that’s not always accurate for eukaryotic cells, which are generally larger than the diameter of the laser focus. Single readings from a cell are affected by factors such as whether the cell is actively replicating DNA, if it’s undergoing apoptosis or necrosis, and the quantity of lipid droplets or proteins. In addition, “you can get variations depending on if you focus the laser on the nucleus or the cytoplasm,” Krafft says. Acquiring multiple spectra from each cell to identify a mean macromolecular signature is ideal, but this is time consuming and more expensive, so researchers may sample fewer cells to compensate.
Solution: Rather than taking multiple individual readings, Schie and Krafft found that scanning a laser beam along an arbitrary line or other defined shape inside a cell can generate a continuous Raman signal from the sample. Most commercial spectrometers are typically developed to analyze materials rather than cells, so the team designed a Raman microscope with several different optical lenses, a multimode optical fiber, motorized table, and other flexible components that make the instrument usable as a microscope as well as for acquiring single point spectra and spectra from multiple points along a line (Biomed Spectrosc Imaging, doi:10.3233/BSI-160141, 2016). The device costs approximately 50,000 Euros ($55,000), unlike commercial instruments that typically run €150,000–€200,000 ($163,000–$217,000), according to Schie.
The team assayed two different pancreatic cancer cell lines and a T-cell line using this setup and compared the results to Raman imaging in which single spectra from many points are combined to produce an image of a cell, and Raman spectra acquired from single points in cells. Integrated spectra yielded less variation between cells of the same type, so fewer cells had to be sampled to train a robust classification model. Using only 20 cells in a training model was sufficient to achieve an accuracy of more than 90 percent. All three methods could distinguish between the two cancer cell lines and the T-cell line. Because taking readings along a line samples cells continuously rather than taking discrete measurements, “the variation between two cells of the same type is not as high as if we acquire single spectra from random sites in cells,” Krafft says. This method could be an effective, high-throughput way to use Raman spectroscopy to detect circulating tumor cells, he adds (Analyst, doi:10.1039/C6AN01018K, 2016).
TRACKING CANCER CELL DEATH
Investigator: Mostafa El-Sayed, professor of chemistry, Georgia Institute of Technology
Project: Using SERS to observe how heated nanoparticles can target and kill tumor cells
Problem: Targeting nanoparticles to tumor markers and heating them with a laser can kill cancer cells, but researchers do not yet know the molecular mechanism. “Understanding precisely how a potential therapy kills cells is important to identify and develop new drugs,” El-Sayed says. Previous explorations have monitored changes in mitochondrial membrane potential using flow cytometry or confocal microscopy, but those methods did not reveal whether the nanoparticles entered cells by passive or active transport, or whether cell death was apoptotic or necrotic. Varying results have been attributed to differences in particle size and shape, or the laser intensity and exposure time used to generate heat.
Solution: Instead of employing an additional method to monitor cellular changes, El-Sayed and his colleagues used actively targeted gold nanoparticles to trigger heat-induced cell death and, with the help of SERS, to monitor how this death occurred in real time. Nanoparticles used for SERS are typically ~50 nm wide, but smaller nanoparticles absorb more radiation than they scatter, converting it to lethal heat, El-Sayed says. So the team used ~29-nm-wide gold particles, coated them with PEG, and linked them to peptides that increase the particles’ uptake into the perinuclear region of tumor cells. They treated cells with a 0.2 nM concentration of nanoparticles, shined a 785 nm laser beam on the sample, and collected SERS spectra for two hours throughout the exposure.
The initial changes in the spectra revealed that laser exposure broke down disulfide bonds in proteins. Later in the process, more protein and lipid structures were disrupted by the laser-induced heat, which eventually caused cells to die. The SERS signatures produced during this process were similar to those seen when cells were killed by heating them in an oven. This trajectory was unrelated to nanoparticle size or laser intensity, but a minimum threshold of particle concentration and laser intensity was needed to spur heat-induced cell death (J Am Chem Soc, doi:10.1021/jacs.5b10997, 2016).
MEASURING MALARIA
Investigator: Quan Liu, assistant professor of bioengineering, Nanyang Technological University, Singapore
 MEASURING MALARIA: Silver nanoparticles (red arrows, left photo) formed in situ near the malaria parasite’s vacuoles produce a stronger SERS signal for hemozoin, a degraded form of hemoglobin, than nanoparticles that are added to lysed blood containing hemozoin released from vacuoles (right). Q. LIU ET AL., SCI REP, doi:10.1038/srep20177, 2016
MEASURING MALARIA: Silver nanoparticles (red arrows, left photo) formed in situ near the malaria parasite’s vacuoles produce a stronger SERS signal for hemozoin, a degraded form of hemoglobin, than nanoparticles that are added to lysed blood containing hemozoin released from vacuoles (right). Q. LIU ET AL., SCI REP, doi:10.1038/srep20177, 2016
Project: Using SERS to diagnose and quantify the severity of malaria
Problem: Field-based, rapid diagnostic tests for malaria are low cost and readily available, but they often lack the sensitivity and specificity needed to detect the small numbers of parasites found in the early stages of infection. Lab-based techniques such as flow cytometry, used to sort infected red blood cells, are more sensitive, but they can only analyze individual or small batches of cells and are primarily used for research. “Each method has its own niche,” says Liu. “We address the [divide] between cheap price and high sensitivity, which is a challenge for most existing methods.”
Solution: During infections, the malaria parasite Plasmodium falciparum feeds on hemoglobin in red blood cells and degrades it in to hemozoin, a molecule that serves as a disease marker and can be detected using Raman spectroscopy. Liu and his team turned to SERS for its high sensitivity, but also because the necessary detection equipment can be built at a low cost, he says.
The researchers used two different methods. In the first, they synthesized silver nanoparticles in the lab and mixed them with hemozoin crystals collected from lysed red blood cells. Hemozoin adhered to the nanoparticles and produced characteristic spectra that could be detected with a sensitivity of 0.01 percent, i.e., approximately 500 parasites/μL of blood. (Flow cytometry, in contrast, has a sensitivity of two percent, or approximately 100,000 parasites/μL.) In the second method, they synthesized nanoparticles inside the parasites themselves. They lysed red blood cells by sonication to release the enclosed parasites, but avoided using the lipid solvent Triton-X, so that the parasites—and their vacuoles containing hemozoin—remained intact. As a result, nanoparticles that formed near or inside these vacuoles adsorbed higher amounts of hemozoin than they did when cells were lysed and hemozoin was released into several milliliters of solution. With this version, the team could detect approximately 2.5 parasites/μL of blood.
The first method caused less variation in SERS measurements, and the correlation between SERS readings and disease severity was stronger. But the second assay was significantly more sensitive to low parasite loads and could be used as an early diagnostic tool, says Liu (Sci Rep, doi:10.1038/srep20177, 2016). Engineering the sample and nanoparticle preparation process to occur on a microfluidic device could turn these assays into a more effective portable, low-cost field test for malaria, he adds.
Interested in reading more?




