 © INGO ARNDT/GETTY IMAGES
© INGO ARNDT/GETTY IMAGES
In a small, pitch-dark room at Baylor College of Medicine in Houston, Texas, a homing pigeon stands on a metal platform, its wings restrained in a leather harness and its head held in place by a plastic arm. The platform sits in the center of a 2-foot cubic frame containing electricity-conducting coils—one on every face of the cube. The coils are programmed to precisely deliver magnetic fields from any direction in three-dimensional space.
In an adjacent room cluttered with racks of whirring, bleeping equipment, neuroscientists David Dickman and Le-Qing Wu stare at their computer screens. The pigeon is stock still as it is subjected to a magnetic stimulus directed from hundreds of different positions: the surrounding coils generate a field that incrementally moves through 360° across 4 different “great circle” planes intersecting the center of the bird’s head. Meanwhile, electrodes implanted in the bird’s brain...
Magnetoreception is a huge mystery. That’s what makes this such an exciting field. We simply don’t know how they do this, so it’s wide open to discovery.
This was Dickman’s and Wu’s daily routine throughout the summer of 2009, as they studied the activity of 329 neurons in the brains of seven different homing pigeons. The researchers were hoping to catch the brain cells in the act of responding to magnetic fields, and thus to better understand how homing pigeons, like many other creatures, use the Earth’s magnetic field for navigation. For months they observed nothing unfamiliar; neurons fired at normal rates, seemingly unaffected by changes in magnetic field. Then, one day, the screen displayed an unusual spiking pattern. “I remember that moment,” says Dickman, who realized then that they’d captured for the first time a neuron responding to information about the bird’s magnetic surroundings. “We were jumping up and down; we broke out the champagne.”
Over the coming weeks and months, Dickman and Wu identified 53 different neurons in the vestibular nuclei, brain regions linked to the inner ear, that are strongly modulated by magnetic stimuli at a range of intensities equivalent to those of the Earth’s magnetic field. Each cell is finely tuned to a field coming from one particular direction. “Let’s say you have a field pointing up from below the bird’s nose to the top of its head at an angle of 45 degrees,” says Dickman. “There is a neuron that is most active in response to that, and least active to a field from the opposite direction, while there is another neuron that responds best to [a field pointing from] the opposite direction.”
They also discovered that the neurons are sensitive to three different aspects of the geomagnetic field: direction, intensity, and polarity. “These are the three components you need to determine your position within the three-dimensional magnetic field,” says Dickman. It is information derived from this tripartite sensitivity, the researchers hypothesize, that homing pigeons might use to pinpoint their position—much as humans use a GPS unit—in addition to using it like a compass, to determine which way they’re heading. “We don’t know that for sure yet,” Dickman adds, “but that’s our guess.”
Although it is a sizable achievement, Dickman and Wu’s discovery does not solve the mystery of magnetoreception. In fact, in at least one way their findings complicate the quest to understand this elusive sixth sense: the study, which was published last year,1 indicates that receptors sensing magnetic information and passing it on to the brain might be found in the inner ear, whereas most researchers have been looking in beaks, snouts, and eyes. A definitive sensor has yet to be found.
Indeed, the question of how animals can detect magnetic fields has proven maddeningly difficult to answer, and scientists are only just beginning to elucidate the physiological mechanisms behind this mysterious extra sense.
“This is a sense that doesn’t exist in humans, so we don’t have any intuitive feeling for what it would be like to perceive magnetic fields,” says Ken Lohmann, a neurobiologist at the University of North Carolina at Chapel Hill, who studies the navigational abilities of sea turtles and other marine animals. “In addition, unlike most other stimuli, magnetic fields go right through biological tissue, so in principle the receptors could be anywhere in the body, and they may not be clustered in one place.”
“[Magnetoreception] is a huge mystery,” says Dickman. “That’s what makes this such an exciting field. We simply don’t know how they do this, so it’s wide open to discovery.”
Magnetic suspicions
 ATTRACTIVE BUG: The bacterium Magnetobacterium bavaricum biomineralizes large amounts of tooth-shaped magnetite crystals (each 100 nm long). Arranged in chains, the magnetite crystals all have consistent magnetic polarity, allowing the cell to swim along magnetic field lines.COURTESY OF MARIANNE HANZLIKFifty years ago, the very idea of magnetoreception was the subject of ridicule. In 1968, Wolfgang Wiltschko, a graduating zoology student at Goethe University in Frankfurt, Germany, demonstrated that the direction in which caged European robins tried to flee in the fall could be changed from its typical southern bearing by using coil systems to simulate magnetic “north” around their enclosure. It was the first real evidence of birds’ internal magnetic compass, but most researchers were skeptical. All previous attempts to prove the existence of such a sense had failed, and many people simply did not believe that it was possible for animals to sense magnetic fields as weak as those generated by the Earth.
ATTRACTIVE BUG: The bacterium Magnetobacterium bavaricum biomineralizes large amounts of tooth-shaped magnetite crystals (each 100 nm long). Arranged in chains, the magnetite crystals all have consistent magnetic polarity, allowing the cell to swim along magnetic field lines.COURTESY OF MARIANNE HANZLIKFifty years ago, the very idea of magnetoreception was the subject of ridicule. In 1968, Wolfgang Wiltschko, a graduating zoology student at Goethe University in Frankfurt, Germany, demonstrated that the direction in which caged European robins tried to flee in the fall could be changed from its typical southern bearing by using coil systems to simulate magnetic “north” around their enclosure. It was the first real evidence of birds’ internal magnetic compass, but most researchers were skeptical. All previous attempts to prove the existence of such a sense had failed, and many people simply did not believe that it was possible for animals to sense magnetic fields as weak as those generated by the Earth.
Today, it is well known that dozens of species, from migratory birds and sea turtles to spiny lobsters and monarch butterflies, have the ability to sense direction and navigate using the Earth’s magnetic field. Not only does the field provide a compass, allowing animals to orient themselves with regard to north and south; it seems that the Earth’s magnetism can also serve as a kind of map.
 MAP LINES: The Earth’s geomagnetic field has two poles—north and south—aligned approximately with the planet’s axis of rotation, much as if a bar magnet was embedded at a slight angle through the center of the Earth. The intensity of the field emanating from the Earth’s surface is strongest at the magnetic poles and weakest at the magnetic equator. The angle at which the field lines intersect the surface, known as the inclination, also varies continuously, from 0° at the magnetic equator to +/−?90° at the poles. Magnetism-sensing animals use these variations in the intensity and inclination of the Earth’s magnetic field for orientation and navigation.© CATHERINE DELPHIAIn the years following his experiments with European robins, Wiltschko teamed up with his wife, Roswitha, also a zoologist at Goethe University, to demonstrate that the birds’ compass sense is based not on the polarity of the Earth’s magnetic field, but on the angles at which the field lines intersect the planet’s surface, known as the inclination. Depending on how the inclination of the field lines change as they fly, the birds perceive whether they are heading poleward or “equator-ward” and gauge their approximate distance between the magnetic equator and the nearest magnetic pole.
MAP LINES: The Earth’s geomagnetic field has two poles—north and south—aligned approximately with the planet’s axis of rotation, much as if a bar magnet was embedded at a slight angle through the center of the Earth. The intensity of the field emanating from the Earth’s surface is strongest at the magnetic poles and weakest at the magnetic equator. The angle at which the field lines intersect the surface, known as the inclination, also varies continuously, from 0° at the magnetic equator to +/−?90° at the poles. Magnetism-sensing animals use these variations in the intensity and inclination of the Earth’s magnetic field for orientation and navigation.© CATHERINE DELPHIAIn the years following his experiments with European robins, Wiltschko teamed up with his wife, Roswitha, also a zoologist at Goethe University, to demonstrate that the birds’ compass sense is based not on the polarity of the Earth’s magnetic field, but on the angles at which the field lines intersect the planet’s surface, known as the inclination. Depending on how the inclination of the field lines change as they fly, the birds perceive whether they are heading poleward or “equator-ward” and gauge their approximate distance between the magnetic equator and the nearest magnetic pole.
More recently, scientists have found that animals also use the Earth’s field lines as magnetic “signposts”—positional information created by unique combinations of field inclination and intensity at specific geographic locations. “What had been overlooked is that those two parameters don’t vary in the exact same direction across the surface of the Earth,” Lohmann says. For example, loggerhead turtles returning to the US coast as juveniles after navigating the North Atlantic gyre—a swirling ocean current—need to adjust their swimming direction at key locations to avoid being swept off course, and Lohmann hypothesized that they might be using the inclination and intensity anomalies to determine where and when to turn.
In 2011 Lohmann and colleagues tested the hypothesis by placing hatchlings in a tank surrounded by magnetic coils and tethering the animals to a swinging metal arm to track their orientation. When hatchlings were exposed to magnetic fields mimicking those found near Puerto Rico or near the Cape Verde Islands, 400 miles off the coast of West Africa—two locations on roughly the same latitude but on opposite sides of the Atlantic—they swam in the appropriate direction to remain within the gyre.2 This was the first evidence that animals can encode information about longitude as well as latitude, and it suggests that the two can be combined in an animal’s brain to pinpoint its specific geographic location.
“We think different areas along the migratory pathway are marked by unique magnetic signatures, and the turtles have evolved responses that are coupled to these signatures,” says Lohmann.
The existence of the signpost sense and the internal compass are now generally accepted, and a number of scientists think that some animals may have both. The sensory and neural mechanisms underlying these senses, however, are more controversial.
A secret sense
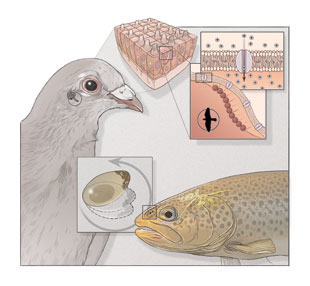
In the mid-1970s researchers discovered that bacteria living at the bottom of the ocean contain microscopic magnetite particles arranged in linear chains inside the cells. (See image on right.) These strings of magnetite—an iron-based substance that is sensitive to magnetic fields—align with the direction and inclination of the Earth’s magnetic field lines, pointing the bacteria toward their preferred habitat in the seafloor sediment.
Nearly 3 decades later, researchers used dyes that bind to iron-based minerals to identify similar-looking iron-rich particles in the upper-beak skin of homing pigeons. By 2010, the particles had also been spotted in the upper beaks of chickens, European robins, and garden warblers. These putative magnetite clusters were located in the dendrites of sensory nerve cells, seemingly integrating them with the central nervous system. What’s more, several studies have suggested that the ophthalmic branch of the trigeminal nerve, which in birds carries signals exclusively from the beak, transmits magnetic information to the brain—so all signs pointed to the iron-based particles in the beak being the magnetic sensors.
But that hypothesis was dealt a severe blow last year, with the publication of a study revealing that the particles in question are in fact contained in immune cells called macrophages,3 rather than in sensory neurons, meaning they are not linked to the brain and are therefore unlikely to be magnetic receptors. “That was quite a big upheaval,” says Michael Winklhofer, a geophysicist at Ludwig-Maximilians University in Munich, Germany, “particularly for people looking for magnetite-based receptors in birds, who have to start all over again.”
A few months later, however, Winklhofer and colleagues identified magnetite-containing cells in the snouts of rainbow trout. The researchers suspended olfactory epithelial cells in liquid and placed them under a microscope around which an artificial magnetic field was rotated—a novel method for testing cellular responses to magnetism. Sure enough, some cells actually rotated with the field. Although these magnetic cells were few and far between—roughly 1 in every 10,000 cells in the olfactory epithelium—their sensitivity to magnetic fields was much greater than expected, suggesting that such cells may be capable of detecting small variations in the geomagnetic field.
Studying these rotating cells one by one under a confocal reflectance microscope, Winklhofer and his team noticed that each one contained a structure of iron-rich crystals, most likely magnetite, attached to the cell membrane.4 Magnetite clusters aligning to the magnetic field in nonmotile cells in the olfactory epithelium of trout might put physical pressure on the cell membrane, he proposes. “When the membrane is stretched, mechanosensitive ion channels may open and change the electric potential across the membrane, which then might trigger a nerve impulse.” (See illustration.) For the moment, though, “the missing link in our story is whether these cells are neurons or not,” says Winklhofer.
Nevertheless, he adds, “the new method is a game changer in that it should allow us to get at these cells more easily and study them in more detail.”
And thanks to Dickman and Wu’s 2012 study, which identified magnetically sensitive neurons in the vestibular nuclei, researchers have expanded the search for magnetism receptors beyond the beak and snout to the inner ear. Indeed, earlier this year another group reported the discovery of a membrane-bound, iron-rich organelle in the cochlear and vestibular hair cells of pigeons and other bird species.5
Magnet eyes
 The Biology of Magnetoreception
The Biology of Magnetoreception
View full size JPG | PDF© CATHERINE DELPHIABut some scientists have an alternative explanation for the biophysical mechanism behind magnetic sensing. In the late 1970s biophysicist Klaus Schulten, then a postdoc at the Max Planck Institute for Biophysical Chemistry in Göttingen, Germany, had an inkling that some bizarre chemical reactions he was studying might form the basis of magnetoreception.
Schulten, now at the University of Illinois at Urbana-Champaign, was looking at reactions involving radical pairs. Electrons normally whirl around their atomic nuclei in even numbers and in set orbits, with pairs of electrons spinning on their axes in opposite directions. But light can disrupt this routine by causing electrons to jump to different regions within the same molecule or to neighboring molecules, resulting in pairs of radicals—molecules with unpaired electrons. In this transient state, the unpaired electrons can spin in one of two ways: in the same direction, known as parallel, or in opposite directions, known as antiparallel—and the amount of time they spend in each state can be influenced by magnetic fields.
These two spin states are chemically different—certain reactions can only take place when unpaired electron spins are parallel and others only when they are antiparallel. Magnetic fields can influence the outcome or speed of chemical reactions involving radical pairs by causing flips between the two spin states and by controlling the relative amount of time the molecular players spend in each. Such fluctuations in reactions could in turn provide a chemical cue about the magnetic field, which could be picked up by a sensory neuron, Schulten thought.
“I had this wonderful proof of a biochemical reaction . . . that was affected in very weak fields, so I thought, well, maybe that’s the internal compass the biologists were looking for,” he says.
 European Robin© ANDREW_HOWE/ISTOCKPHOTO.COMIn 1978, Schulten submitted a paper detailing his theory to Science—and promptly received a rejection note that read, “A less bold scientist would have designated this piece of work for the waste paper basket.” Undeterred, Schulten published in an obscure German journal. Since the formation of radical pairs in vitro required light, he thought the most likely site for the interaction in a living creature would be the eye. But at the time, there was no known photoreceptor in the eye capable of forming radical pairs.
European Robin© ANDREW_HOWE/ISTOCKPHOTO.COMIn 1978, Schulten submitted a paper detailing his theory to Science—and promptly received a rejection note that read, “A less bold scientist would have designated this piece of work for the waste paper basket.” Undeterred, Schulten published in an obscure German journal. Since the formation of radical pairs in vitro required light, he thought the most likely site for the interaction in a living creature would be the eye. But at the time, there was no known photoreceptor in the eye capable of forming radical pairs.
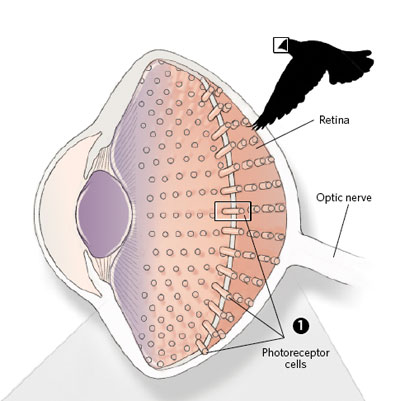
That changed in 1998, with the discovery of a blue-light photoreceptor protein that generates radical pairs when exposed to light. Known as a cryptochrome, the protein has since been identified in the retinal tissue of several migratory birds and of other animals, including humans. Assuming that the products of radical-pair reactions in cryptochromes could in some way affect the sensitivity of light receptors in the retina, Schulten and Thorsten Ritz, a biophysicist now at the University of California, Irvine, argued in a theoretical paper published in 2000 that the modulation of these reactions by magnetic fields would affect the visual system of birds to provide compass cues.6 (See illustration.)
In the intervening years, researchers have produced reams of circumstantial evidence in support of this idea. In 2004, for example, Ritz and the Wiltschkos showed that high-frequency radio waves, known to disrupt the spin behavior of radical pairs in vitro, stopped robins from navigating.7 Three years later, Henrik Mouritsen, a neurobiologist at the University of Oldenburg in Germany, and colleagues showed that a cryptochrome isolated from the garden warbler produces long-lived radical pairs.8 And in 2011, the Wiltschkos showed that one of the four known bird cryptochromes is found in all ultraviolet/violet cone cells in the retinas of both European robins and chickens—specifically, in the stacked membrane discs of the photosensitive outer segment of the cone cell, which also contains the visual pigments.9
“What we have is a lot of evidence that the necessary conditions for this hypothesis to be true are in place,” says Ritz. “But then you need to connect these findings with the behavior of the animals, and that’s where we’re faced with a big gap.”
Some of the most compelling evidence for the importance of cryptochromes came in 2008, when Steven Reppert, a neurobiologist at the University of Massachusetts Medical School in Worcester, and colleagues showed that wild-type fruit flies responded to magnetic fields under full-spectrum light—they could even be trained to follow artificial magnetic cues to find food—but when the team blocked the particular wavelengths of light to which cryptochromes are sensitive, the same flies could no longer detect magnetic stimuli. Furthermore, mutant flies lacking the gene for cryptochrome did not respond to magnetic fields even under full-spectrum light,10 and in 2010, Reppert rescued the magnetosensory ability in mutant flies by transfecting them with two different cryptochrome genes from monarch butterflies.11 “The deletion and replacement experiments really said that the cryptochrome is an essential feature of the light-dependent magnetic sensing system in Drosophila,” he says.
But the case is far from closed. First of all, Reppert’s flies were exposed to magnetic fields 8 to 10 times stronger than the one emanating from the Earth. Moreover, Reppert says that knockout experiments on migratory animals would provide more compelling evidence—and it’s important that the results are replicated with individual animals that routinely reproduce magnetic orientation behavior in the lab rather than the population-based studies his group performed with fruit flies. Unfortunately, that’s unlikely to be achieved with birds or turtles in the near future because of the difficulties associated with creating gene knockouts and transgenic lines in such animals, but Reppert says his group is “making tremendous progress” with monarch butterflies, whose genomes can be manipulated.
Proponents of the cryptochrome theory also have yet to show that retinal neurons respond to variations in Earth-strength magnetic fields; to elucidate the signaling pathways that connect cryptochromes to the nervous system; or to explain how animals that possess this visually mediated compass perceive the information it provides—though some have speculated that the magnetic field might appear as patterns of light and shade superimposed on the animals’ vision.
Navigating the future
 Compass Eyes
Compass Eyes
View full size JPG | PDF© CATHERINE DELPHIADespite recent progress, the field of magnetoreception has been hindered by the fact that many findings have proven very difficult to replicate, says Mouritsen. “The field has been slowed down by a number of claims that have turned out not to be right,” he says. One reason for this irreproducibility is that humans lack the magnetic sense, so researchers are susceptible to misinterpreting artifacts as real results. “It will be helped a lot if everybody would start doing double-blind experiments with very carefully controlled and measured stimuli,” adds Mouritsen. “If that happens we would move forward much faster.”
As it stands, there are several hypotheses supported by strong, albeit not yet conclusive, data. Most researchers in the field agree that the compass sense is likely seated in cryptochromes within the eye, and many are convinced that there is another sense, most likely a signpost sense, passed through the trigeminal nerve and probably based on some sort of iron-containing, magnetism-sensing cells in beaks or snouts. Then there is Dickman and Wu’s idea: that both of these abilities may rely on receptors in the inner ear.
Moreover, Dickman points out, these mechanisms may not be mutually exclusive. “It could be that there are multiple [receptor] sites that may be providing different sorts of information about different aspects of the magnetic field, which then converge in the brain,” he says.
Although the researchers who have come together from a diverse range of disciplines to unravel the secrets of magnetoreception are still some way from their final destination, there is a palpable sense of momentum. “It’s really tough work, but it’s also exciting,” says Winklhofer, “because everybody wants to be the first to demonstrate how this extra sense works.”
References
- L.Q. Wu, J.D. Dickman, “Neural correlates of a magnetic sense,” Science, 336:1054-57, 2012.
- N.F. Putman et al., “Longitude perception and bicoordinate magnetic maps in sea turtles,” Curr Biol, 21:463-66, 2011.
- C.D. Treiber et al., “Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons,” Nature, 484:367-70, 2012.
- S.H.K. Eder et al., “Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells,” PNAS, 109:12022-27, 2012
- M. Lauwers et al., “An iron-rich organelle in the cuticular plate of avian hair cells,” Curr Biol, 23:924-29, 2013.
- T. Ritz et al., “A model for photoreceptor-based magnetoreception in birds,” Biophysical Journal, 78:707-18, 2000.
- T. Ritz et al., “Resonance effects indicate a radical pair mechanism for avian magnetic compass,” Nature, 429:177-80, 2004.
- M. Liedvogel et al., “Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical pairs,” PLOS ONE, 2:e1106, 2007.
- C. Niessner et al., “Avian ultraviolet/violet cones identified as probable magnetoreceptors,” PLOS ONE, 6:e20091, 2011.
- R.J. Gegear et al., “Cryptochrome mediates light-dependent magnetosensitivity in Drosophila,” Nature, 454:1014-18, 2008.
- R.J. Gegear et al., “Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism,” Nature, 463:804-07, 2010.
Interested in reading more?




