It started with a picture,” says University of Colorado Boulder molecular and cellular biologist Gia Voeltz. When she was still a postdoc, Voeltz and her colleagues started painstakingly assembling 3-D electron microscopy and tomography images of the endoplasmic reticulum (ER) in hopes of locating a structural protein on its surface.
But as soon as they had the assembled images, “we started to get a glimpse of all these interactions” of the ER with other organelles, most notably with mitochondria, and their interest in reticulin proteins fell by the wayside, she says. “There were these contacts where the ER tubes were literally wrapping around the mitochondrial membrane.” When they looked more closely, it appeared that the ER was constricting the mitochondria at some of its contact points.
Most researchers agree that mitochondria divide with the help of a highly conserved cellular protein called dynamin-related protein (Drp1; Dnm1 in yeast), oligomers...
To catch the organelles in action, they fluorescently tagged the mitochondria, endoplasmic reticulum, and dynamin-related protein with different colors and used a confocal fluorescence microscope to shoot videos of live mammalian and yeast cells. They recorded an intricate dance: the ER encircled the mitochondria at narrow points, and then Drp spiraled around the mitochondria at those spots to trigger division. The images of ER also helped explain how Drp, which is a rigid, helical protein with a much smaller relative diameter, managed to wrap itself around the bulkier mitochondria “like stripes on a barber’s pole”—by slipping around the mitochondria specifically at those narrow spots marked by the ER, Voeltz says.
The “incredibly elegant” paper is one of the first to show that organelles interact and are not just “swimming in a sea of cytoplasm,” says Ruth Collins, a molecular cell biologist at Cornell University. The imaging study also raises a host of new questions, she says. The authors speculate that the ER literally squeezes the mitochondria at contact points, but another possibility is that the mitochondria are somehow pre-cinched before the ER gets there. After determining that, the next big question will be how exactly the ER and the mitochondria communicate to determine the spots of division. Voeltz’s group plans to study just that.
The study also raises the question of what other organelles are doing with each other when the lights are out. Just how they communicate to orchestrate these dances is “really a wide-open question,” Collins says.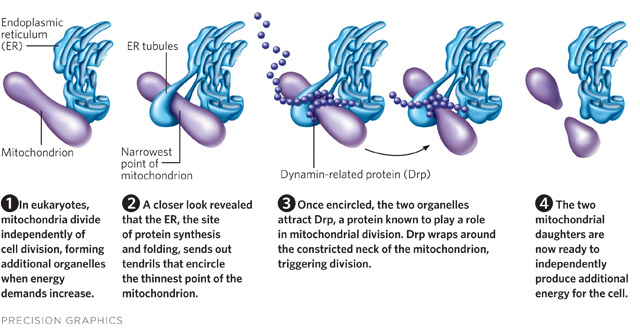
The paper
ScienceInterested in reading more?




