Current hypotheses suggest that all life on Earth evolved from a common ancestor that diversified into the variety of organisms seen today. Scientists propose that the eukaryotic branch of this family tree formed when two prokaryotes, an ancient bacterium and archaeal cell, merged, forming a symbiotic relationship maintained over time.1
“If this is true,” said Pedro Leão, an archaeal biologist at Radboud University, “we have to understand what types of features this [archaeal] host was bringing to the first eukaryote.” As a postdoctoral researcher in Brett Baker’s lab at the University of Texas at Austin, Leão explored the archaeal origins of eukaryotes, or eukaryogenesis.
Leão stumbled upon prokaryotic defense systems after he and his colleagues identified archaea-infecting viruses.2 He reasoned that archaea, like bacteria, would also possess antiviral mechanisms, and these could have been preserved in modern eukaryotes. He started digging into the literature in search of information on archaeal defense systems and the origins of eukaryotic immunity, and came across a perspective paper that piqued his interest: It attributed eukaryotic immune components predominantly to bacteria and only briefly mentioned the existence of archaeal defense proteins.3
Leão realized that one reason for this archaeal slight was that archaeal genomes were poorly represented in the studies that investigated the prokaryotic origins of eukaryotic defense systems.4,5 “I was like, ‘Okay, this is an easy job for what we do. We can just curate a good dataset of archaea genomes and look for exactly the same things,’” Leão recalled.
He and his colleagues explored the archaeal defense systems of Asgard archaea, the closest modern prokaryotic relative to eukaryotes, and compared their homology to those of eukaryotes. In a paper published in Nature Communications, the team demonstrated that two classes of defense system proteins found in these archaea are related to those of eukaryotes.6 The findings offer deeper insight into the origins of early immune systems and how they functioned.
Leão and his team used a database of prokaryotic defense systems to study the distribution of complete systems in the genomes of archaea, including Asgard archaea, and bacteria.7 The researchers identified two groups of proteins that were more common in Asgard archaea than in other archaea and bacteria: argonautes, proteins from the RNA-induced silencing complex, and viperins (short for virus-inhibitory protein, endoplasmic reticulum-associated, interferon inducible).
Viperins and argonautes are both highly represented in eukaryotic antiviral defense systems, so the team compared these proteins’ similarity to those found in Asgard archaea.8,9 They constructed a phylogenetic tree using eukaryotic, archaeal, and bacterial genomes and showed that both viperin and argonaute proteins in eukaryotes shared an ancestor with those from Asgard archaea. They further confirmed that the structural homology, including key functional domains, of both proteins was preserved between archaea and eukaryotes.
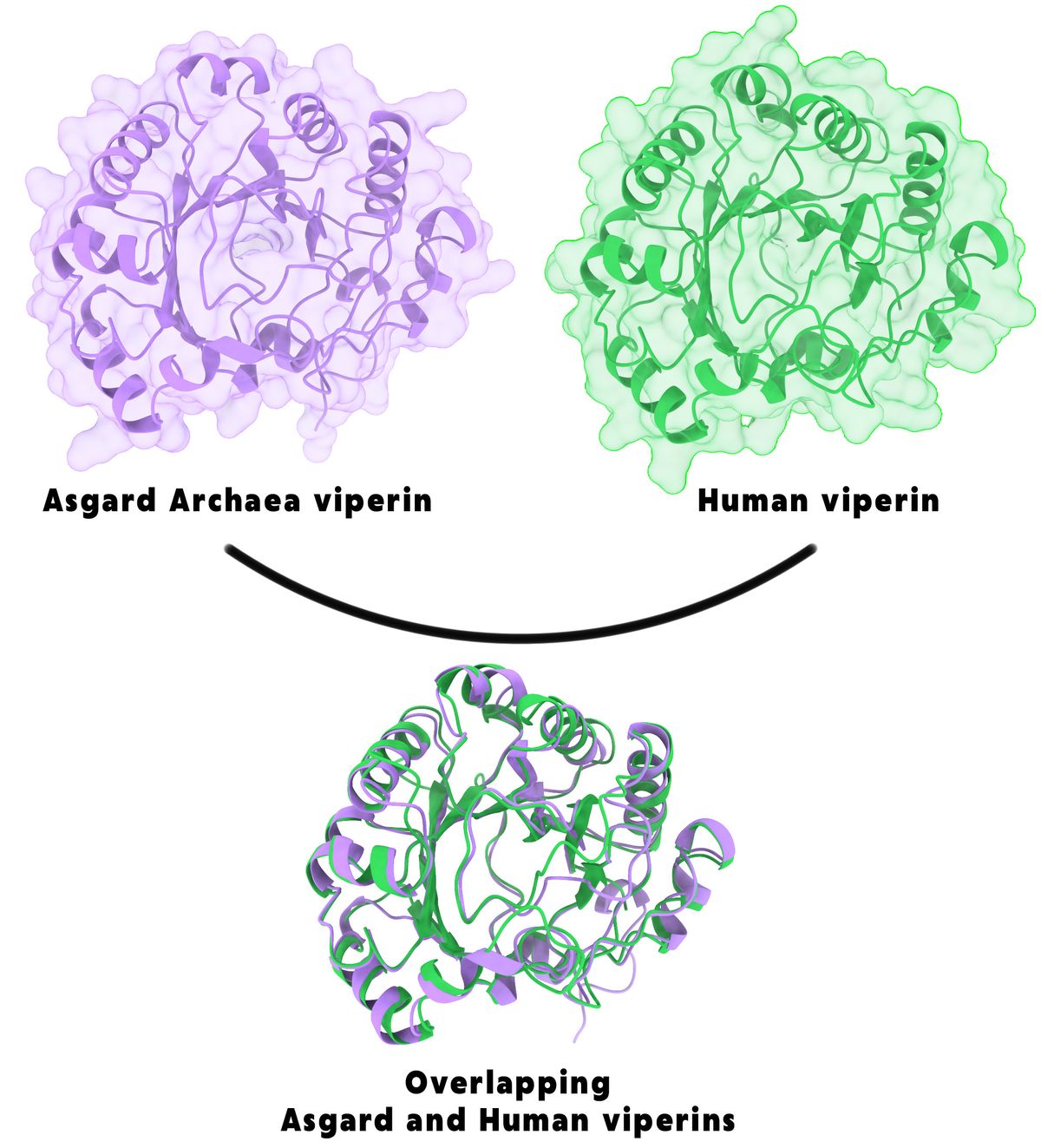
While the shared sequence and structure homology of these proteins was promising, Leão sought to further demonstrate that these archaeal proteins functioned like those of eukaryotes. The team expressed 48 different Asgard viperin genes in Escherichia coli before introducing bacteriophages to evaluate the viperins activity. Of the proteins produced, nine protected E. coli against virus infection. Since some sequences were more similar to those of eukaryotes, the researchers optimized the viperin genes that encoded these nine proteins for expression in E. coli, and one additional archaeal viperin demonstrated anti-phage activity.
The team also found that, on average, Asgard archaea have a comparable number of defense systems per genome to other archaea and bacteria. However, some classes had a much lower ratio, including Heimdallarchaeia, which is most similar to eukaryotes.
“In my mind, this is a good tip that we might have some unique defense systems just in archaea in general,” Leão said. He intends to explore archaea-specific defense systems in more detail in his lab. “If we keep looking for defense systems in Archaea through homologs of already described ones in bacteria, we won't find them.”
Buzz Baum, a cell biologist studying archaea at the Medical Research Council Laboratory of Molecular Biology who was not involved in the study, shared this thought. “There might be lots of things in Asgard which we don't know what they do, but actually do function in [an] immune context.” He is also excited to learn more about how the archaeal immune system functions. “It will be wonderful in the coming years to be able to see, in real time, how cells employ these systems to defend themselves.”
- Martin WF, et al. Endosymbiotic theories for eukaryote origin. Philos Trans R Soc B. 2015;370(1678):20140330.
- Rambo IM, et al. Genomes of six viruses that infect Asgard archaea from deep-sea sediments. Nat Microbiol. 2022;7(7):953-961.
- Wein T, Sorek R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat Rev Immunol. 2022;22(10):629-638.
- Bernheim A, et al. Prokaryotic viperins produce diverse antiviral molecules. Nature. 2021;589(7840):120-124.
- Swarts DC, et al. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol. 2014;21(9):743-753.
- Leão P, et al. Asgard archaea defense systems and their roles in the origin of eukaryotic immunity. Nat Commun. 2024;15(1):6386.
- Tesson F, et al. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat Commun. 2022;13(1):2561.
- Rivera-Serrano EE, et al. Viperin reveals its true function. Annu Rev Virol. 2020;7(1):421-446.
- Bartel DP. MicroRNAs. Cell. 2004;116(2):281-297.















