Millions of people across the globe share a grim commonality: They all have end-stage organ failure. Many will die waiting for human organs to become available. “The only solution, currently, is to use animal organs,” said Mike Curtis, chief executive officer of biotechnology company eGenesis. Xenotransplantation has had a controversial history, but Curtis and other experts firmly believe they are about to pass the final hurdle.
So, what is xenotransplantation? Xenotransplantation, or xenograft, is the transplantation of organs, cells, or tissues from one species to another.1 It may sound like a recent development bordering on science fiction, but as University of Sydney transplant physician Wayne Hawthorne remarked: “People have been trying to do these sorts of things forever.”
The Bizarre History of Xenotransplantation
The first documented xenograft took place in 1667, when French surgeon Jean-Baptiste Denis transferred whole blood from a lamb to a 15-year-old.1 Remarkably, the patient survived, but it was far from a triumph. “He didn't get much blood infused,” explained Hawthorne. “[Jean-Baptiste] subsequently went on to transfuse a couple of other patients and killed them.”
In the next few centuries, xenotransplantation attempts ranged from the bizarre to the grotesque: In the early 1800s, frogs were sometimes skinned alive to provide xenografts for the healing of human ulcers, and the first corneal xenotransplant from pig to human was performed in 1838—more than 65 years before a human-to-human corneal transplant was even attempted.2 In the 1920s, slices of chimpanzee testes were transplanted into elderly men under the hypothesis that the hormones they produced would encourage ‘rejuvenation’.
Since then, progress in the xenotransplantation field has come in fits and starts. Chimp testes aside, the early 1900s saw numerous attempts at organ xenotransplantation with varying degrees of success; patients who received organs from baboons, chimpanzees, rabbits, sheep, and pigs died within hours to days. The inevitable rejection of animal organs by the human immune system seemed an insurmountable obstacle, and for 40 years, xenograft research came to a complete halt.
Overcoming the Challenges of Xenotransplantation: Immunosuppression, Retroviruses, and Gene Editing
In the 1960s, the development of immunosuppression techniques led to renewed interest in xenotransplantation. A patient on an intensive immunosuppression regimen received a kidney from a rhesus monkey and survived for 63 days, after which he died of pneumonia. Other kidney xenotransplant patients survived up to nine months, while a heart xenotransplant failed within two hours.
Over the next several decades, scientists learned from each attempt, and ethical and regulatory views of the research evolved. In the late 1990s, the FDA announced a ban on clinical trials involving xenotransplantation from non-human primates (NHPs) to humans, after they determined the risk of zoonotic transmission of viruses was too high.3 Pigs were the next best candidates; they had been used to produce insulin for years, and they could easily be scaled up.
That same decade, researchers identified what they believed were the major antigens responsible for the rejection of xenografts from pigs. “What we learned was that what we call hyperacute rejection is primarily driven by carbohydrate differences between pigs and humans,” explained Curtis. “By removing those carbohydrate antigens through editing, we can eliminate hyperacute rejection.”

A gene-edited Yucatan minipig created by Massachusetts-based xenotransplantation company eGenesis.
Liz Linder, eGenesis
Unfortunately, researchers quickly discovered another major obstacle: porcine endogenous retroviruses.4 These viruses could potentially infect humans, and pigs can carry up to 70 copies in their genomes. “We were just coming off of our understanding of HIV, and the last thing anyone wanted to do was release another retrovirus into the human population,” Curtis said.
No one knew what to do about the challenges of xenotransplantation and the retroviral transmission risk until the development of CRISPR gene editing technology in 2012. Researchers could use CRISPR to eliminate multiple copies of viral DNA sequences throughout the pig genome, and they could also knock out the carbohydrate antigens responsible for immune rejection and knock in several regulatory human transgenes. It is also used to inactivate the growth hormone receptor, preventing pig organs from becoming too large to be transplanted into humans.
Mixed Results in Heart Xenotransplantation
In 2022, a heart xenotransplant from a gene-edited pig was performed at the University of Maryland School of Medicine, spearheaded by pioneer Muhammed Mohiuddin. For more than one month, the xenograft appeared to function normally with no evidence of rejection, but the 57-year-old patient experienced complications at day 49 post-transplant and later died.5 A second patient received a pig heart xenotransplant in 2023, and again, the organ functioned well for several weeks before the 58-year-old patient developed complications and passed away.6
Subsequent investigations revealed several factors that contributed to the patients’ deaths. In the first, there was reactivation and replication of latent porcine cytomegalovirus—which the pre-surgery screening had missed—which potentially caused damaging inflammatory responses.7 Studies are ongoing for the second patient, but there was no evidence of viral replication. In both cases, antibody-mediated rejection contributed to graft failure, but not hyperacute rejection (which occurs within minutes). “Those two patients were also incredibly sick,” Curtis added. “The chances of them recovering were pretty slim.”
Hawthorne said the public view of these heart xenotransplants as failures misses the mark: “[The second patient’s] son came along to our xenotransplant conference and gave an amazing speech, saying how grateful he and his family are for getting his dad for another couple of months, which he would never have had. He was going to die within two to three days,” he said.
Lung Xenotransplantation in Brain-Dead Patients
Researchers have also turned to brain-dead patients to help them understand the risks and feasibility of xenotransplantation. A recent study published in Nature Medicine described the transplant of a genetically modified pig lung into a 39-year-old brain-dead patient which functioned for nine days.8
Unlike other organs, the lungs pose unique challenges for researchers. Aside from their physiological complexity, the lungs are constantly exposed to the external environment, increasing the risk of infection and immune complications. High blood flow to the lungs also increases the chances of ischemia-reperfusion injury, in which the sudden rush of blood to the tissue after oxygen starvation causes damage to the transplanted organ.
The authors of the study reported that, although there were no signs of hyperacute rejection, infection, or retroviruses, antibody-mediated rejection ultimately led to the failure of the lung xenotransplant.
Kidney Xenotransplants: A New Era?
In 2022, researchers from the University of Alabama at Birmingham transplanted pig kidneys with 10 gene edits into a brain-dead patient; the proof-of-concept study demonstrated that the organs remained viable for 77 hours, after which the study was ended. More recently, eGenesis has provided genetically modified pigs for several kidney xenotransplants into living patients. These organs have been reported to have a staggering number of edits, but as Curtis explained, the majority of those 60+ genomic alterations are deletions of integrated retroviruses.
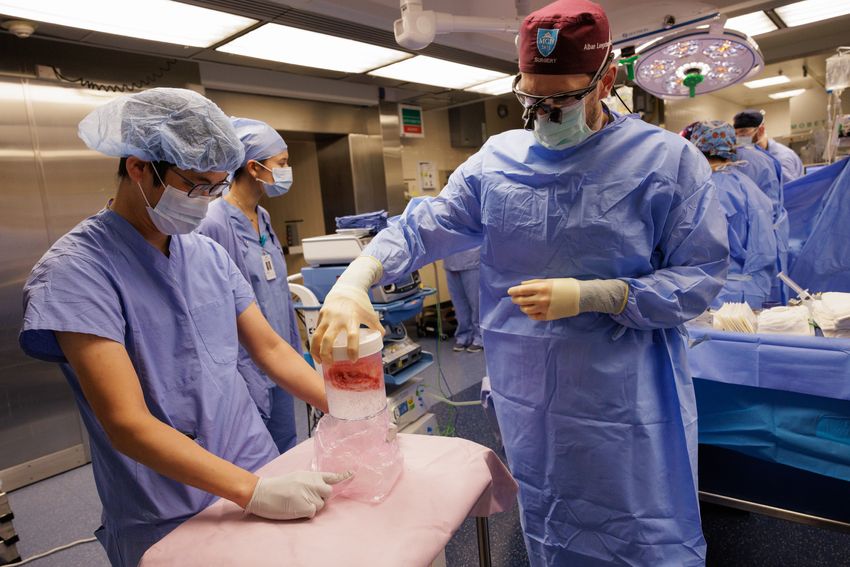
66-year-old Tim Andrews, who had end-stage kidney disease, received a pig kidney xenotransplant with multiple genomic edits.
Kate Flock, Massachusetts General Hospital
Using their gene editing platform, the eGenesis team performs the multiple necessary edits on a wild-type pig cell, then selects clones that have the full complement of edits to create a cell bank. “We take that parental cell, we do axon nuclear transfer, similar technology that was used to clone Dolly [the sheep] in the 1990s, and then we make piglets from those cells,” Curtis said. “So we really only need to edit, in essence, once, and once we have the edited cell line, we can make pigs indefinitely.”
The kidneys provided by the company have had varying degrees of success. One patient died two months post-surgery from unrelated causes, while another had limited blood flow and organ failure. A third patient received a kidney that functioned for more than four months, but later experienced immune rejection.
Then came Tim Andrews, a 69-year-old with diabetes and end-stage kidney failure. Andrews, who went to lengths to improve his health before surgery, received a kidney xenotransplant in January 2025. He is now the longest-living recipient of a pig kidney, and his health has improved dramatically. “He was on dialysis, and he had progressed into a wheelchair, so he was no longer ambulatory, and the week after his kidney transplant, he was up walking out of the hospital,” Curtis commented.
While Andrews is still on the waitlist for a human organ, Curtis said this shouldn’t be an indicator that pig organs aren’t a viable long-term option. Rather, the goal of kidney xenotransplantation is to get patients off dialysis. eGenesis has been working alongside the FDA for several years to address safety concerns, including retroviral transmission risk and the robustness of gene editing in the donor pigs.
The company also has liver and heart xenotransplantation programs, and plans to break ground on a scaled-up donor production facility next year, with the hope of finally putting xenotransplantation within reach for the millions of patients who need new organs. eGenesis recently announced IND clearance for a phase 1/2/3 study that will assess the safety and efficacy of their EGEN-2784 triple-edited pig kidneys at 24 weeks post-transplant. “Now it's a matter of running more patients through,” said Curtis. “And our goal over the next couple of years—between now and next year—is to treat over 20 patients.”
Hawthorne, who continues to push the field of islet cell and kidney xenotransplantation research forward in Australia, concluded that every step forward is a learning opportunity and a success for the field. “Every time we are transplanting a human patient, we are gaining something for society and those patients that require a transplant, and our knowledge base of what we can do to improve things,” he said.
Xenotransplantation: Frequently Asked Questions
Xenotransplantation definition
Xenotransplantation, or xenograft, is a procedure in which cells, tissues, or whole organs are transplanted from a donor animal into a human patient, typically in patients with end-stage organ failure. The most common donor animals are pigs. While there are still some obstacles to their widespread use, the success of a recent pig-to-human kidney xenotransplant has led to renewed interest.
What was the Maryland xenotransplant?
The Maryland xenotransplant refers to two heart xenotransplants performed at the University of Maryland School of Medicine in 2022. Led by pioneer Muhammed Mohiuddin, both xenotransplantations were successful and the organs functioned well for several weeks. However, both patients developed complications relating to antibody-mediated rejection and later died. The presence of latent porcine cytomegalovirus in one patient’s donated heart may have also contributed to inflammation and organ failure.
Why was the Tim Andrews xenotransplant significant?
Tim Andrews’ xenotransplant was a breakthrough in the field and is considered a major success. Andrews, a 69-year-old patient with diabetic kidney disease, received a gene-edited pig kidney xenotransplant in January 2025. While he remains on the waitlist for a human organ, the procedure saved Andrews’ life and he is now the longest-living patient to receive a pig kidney.
Xenotransplants pros and cons
While it might seem macabre, xenotransplantation can have benefits for a range of patients. Perhaps the most obvious is that if successful, the procedure will allow those with end-stage organ failure to live longer, even while they remain on the waitlist for a human organ. For patients with kidney failure, xenotransplantation would free them from the exhausting and time-consuming process of dialysis.
However, both the animal organs and the xenotransplantation procedure itself also carry a number of potential downsides. Xenotransplantation risks include contamination with porcine endogenous retroviruses, inflammation, and immune rejection. Additionally, only the sickest patients typically volunteer to have xenotransplantation procedures, meaning they are less likely to survive the already risky major surgery.
Ethical issues in xenotransplantation
There are a number of ethical issues in xenotransplantation. In the late 1990s, the FDA placed a ban on the use of non-human primates in xenotransplantation research after they determined there were significant risks associated with the zoonotic transmission of disease between primates and humans.
The rearing of non-human primates in captivity for such procedures is also costly and ethically fraught; they breed slowly, cannot be kept in tightly controlled conditions, and some are endangered species. As such, many governments and regulatory bodies have strongly discouraged or banned the use of primates in xenotransplantation research.
Pigs are currently used as the main source of organs for xenotransplantation, as they have short gestation times, relatively large litter size, and can be kept in more controlled laboratory conditions. However, animal rights activists have voiced their objections to the use of animals as organ donors, and some groups object to xenotransplantation on religious grounds.
Siems C, et al. Brief history of xenotransplantation. Ann Thorac Surg. 2022;113(3):706-710.
- Cooper DKC, et al. A brief history of clinical xenotransplantation. Int J Surg Lond Engl. 2015;23(0 0):205-210.
- Butler D. FDA warns on primate xenotransplants. Nature. 1999;398(6728):549-549.
- Denner J. Porcine endogenous retroviruses and xenotransplantation, 2021. Viruses. 2021;13(11):2156.
- Griffith BP, et al. Genetically modified porcine-to-human cardiac xenotransplantation. N Engl J Med. 2022;387(1):35-44.
- Griffith BP, et al. Transplantation of a genetically modified porcine heart into a live human. Nat Med. 2025;31(2):589-598.
- Mohiuddin MM, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. The Lancet. 2023;402(10399):397-410.
- He J, et al. Pig-to-human lung xenotransplantation into a brain-dead recipient. Nat Med. Published online August 25, 2025:1-6.