 |
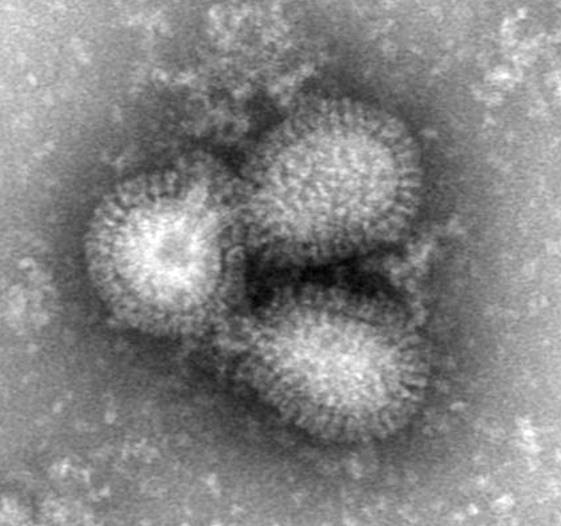
Image: Novavax, Inc. |
**__Related stories:__***linkurl:Vaccine dreams;http://www.the-scientist.com/article/display/54882/
[August 2008]*linkurl:From SARS to avian flu;http://www.the-scientist.com/article/display/15315/
[14th March 2005]*linkurl:Playing chicken with flu;http://www.the-scientist.com/article/display/15152/
[20th December 2004]













