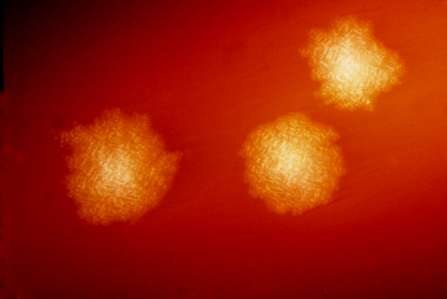
 Clostridium difficile colonies on blood agarCDC, HOLDEMANClostridium difficile (C. diff ) is one of the most common hospital-acquired infections, in part because of its ability to opportunistically colonize the GI tract after antibiotics have disrupted the natural balance of the gut microbiome. But results from a Phase 2 clinical trial announced yesterday (January 5) suggest that Synthetic Biologics’s SYN-004 (ribaxamase) may help degrade certain antibiotics within the GI tract, maintaining the status quo in the gut microbiome and preventing C. diff infection.
Clostridium difficile colonies on blood agarCDC, HOLDEMANClostridium difficile (C. diff ) is one of the most common hospital-acquired infections, in part because of its ability to opportunistically colonize the GI tract after antibiotics have disrupted the natural balance of the gut microbiome. But results from a Phase 2 clinical trial announced yesterday (January 5) suggest that Synthetic Biologics’s SYN-004 (ribaxamase) may help degrade certain antibiotics within the GI tract, maintaining the status quo in the gut microbiome and preventing C. diff infection.
The randomized, double-blind trial involved 412 patients, half of whom received 150 mg of ribaxamase and half of whom received a placebo. By the end of the trial, there were seven cases of C. diff infection in the placebo group but only two in the test group, representing a 71.4 percent relative risk reduction.
The successful trial represents “a significant milestone in the clinical development of ribaxamase,” Jeffrey Riley, president and CEO of Synthetic Biologics, said in a press release. “These findings also help further our goals to bring the first ever microbiome-focused therapeutic to patients and to help illuminate the potential of this drug class to address serious diseases and public health concerns.”
Ribaxamase, an oral enzyme, targets beta-lactam antibiotics, which can save lives ...



















