A moose in Minnesota stumbles onto the road. She circles, confused and dazed, unable to orient herself or recognize the danger of an oncoming semitruck. What kills her is the impact of 13 tons of steel, but what causes her death is more complicated. Tunneling through her brain is a worm that doomed both of them to die.
Commonly known as the brain worm, Parelaphostrongylus tenuis is a parasitic nematode that infects a large range of wild and domestic herbivores, such as moose and elk. The worm can migrate into the brain of unsuspecting hosts, where it may cause catastrophic disease and death.
While the Minnesotan moose is a hypothetical example, this worm has caused serious neurological impairments in many animals. The symptoms of the disease can vary, from disorientation and circling to paralysis across the animal’s back end, the inability to stand up and potentially death.
As parasitologists, we’ve been studying the effects these worms can have on moose populations in Minnesota. Tracking the spread of parasites and diseases in wild moose populations helps wildlife managers preserve those populations and reduce the spread to other animals or livestock.
While white-tailed deer can harbor these parasites without having any symptoms of disease, the worm can wreak havoc on populations of ungulates, like moose and elk, that aren’t adapted to the parasite. And tracking the disease in the wild isn’t easy.
The Disease Cycle
White-tailed deer harboring these parasites may shed the worms into their environment when they defecate. Snails and slugs then take up this larva, where it develops inside them to the point where it’s capable of infecting other types of deer, moose, elk and cattle.
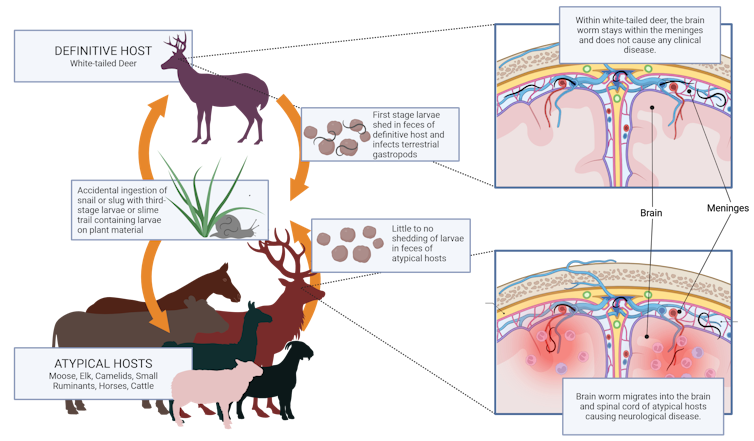
For us as parasitologists, the biggest challenge lies in detecting the disease before it irreversibly damages its host. Only white-tailed deer pass the parasite in their feces. This means we can’t detect this parasite by analyzing the poop of moose, or any animal, besides the white-tailed deer.
Once an animal is visibly sick, it’s too late for it to make a recovery. Only after their death can we recover the body and identify the parasite from where it’s embedded in the brain or spinal cord.
Even once we’ve recovered the body, finding a single, threadlike worm within the entirety of a moose or elk’s nervous system is time-consuming and often futile. Usually, wildlife biologists can only tell that an animal was infected by looking at microscopic evidence that suggests a parasite migrated through the central nervous system, and by analyzing DNA fragments left behind by the worm.
Diagnostic Confusion
To make things even harder, disease signs caused by other worms, like the arterial worm Elaeophora schneideri, look similar to brain worm and can affect Minnesota moose. The arterial worm generally lives in the neck of black-tailed deer and mule deer. Like P. tenuis, this parasite moves around in the bodies of hosts that aren’t adapted to it, and can cause harm.
Biologists attempting to diagnose a wild moose based on the visible clinical signs alone could easily confuse these two parasites and incorrectly conclude which parasite may have caused the disease. Given that the transmission of the parasites are vastly different, separate mitigation steps would be employed to minimize transmission.
And, biologists diagnosing based on microscopic findings in samples from the animal’s body still risk misidentifying the worm. The best way to get an accurate diagnosis is through genetic analysis – analyzing the DNA sequence of the worm causing disease.1 The DNA sequence will tell researchers whether it is P. tenuis or E. schneideri.
Serological Testing
While genetic analysis can help researchers monitor the presence of the disease in a population, they can’t use it to diagnose live animals. But our team, with colleagues at the University of Tennessee College of Veterinary Medicine’s molecular diagnostic lab, has created a test that can help diagnose animals while they’re alive.2
When a moose or elk has a brain worm, its cells produce antibodies, which are a type of protein in the blood that try to defend against the parasite.3 Our serological test looks for these antibodies in an animal’s blood.
To perform the testing, wildlife health specialists collect blood from sick or recently deceased animals and ship it to the lab. There, scientists run part of the blood through a test that looks for these specific antibodies against P. tenuis, so the animal isn’t misdiagnosed with another type of parasite.
This test, which the molecular diagnostic lab is now using to test samples sent in from across the country, has helped us monitor populations of moose and elk for this parasite. It can detect the parasite’s presence while the animals are still alive and without expensive genetic testing.
Ripple Effects from Testing
After the Minnesotan moose from our example is hit by a semitruck, wildlife officials find the deceased moose on the side of the road and quickly take a sample of her blood for testing. They send it off to the University of Tennessee, where it joins thousands of other samples from moose, elk and even caribou across North America.
Each submission helps our colleagues in the molecular diagnostic lab improve the test. The test can also screen blood samples from animals that live in areas where researchers haven’t detected P. tenuis. If positive, those results may alert biologists that the parasite is expanding into new areas and help them manage populations.
If a test at the molecular diagnostic lab indicates that the parasite is present in a new population early on, they will have more time to try to curb the disease spread. Wildlife managers may try to reduce snail and slug populations with controlled burns. Or, they might increase how many white-tailed deer hunters in the area can harvest to reduce the deer population.
We hope that in the future, other researchers will use the techniques behind this serological test to make similar tests for other infectious disease agents containing RNA or DNA.![]()
Written by: Richard Gerhold, Professor of Parasitology, University of Tennessee and Jessie Richards, PhD Student in Parasitology, University of Tennessee
This article is republished from The Conversation under a Creative Commons license. Read the original article.
- Genetic Alliance; The New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services. Understanding Genetics: A New York, Mid-Atlantic Guide for Patients and Health Professionals. APPENDIX G, GENETIC TESTING. Washington (DC): Genetic Alliance; 2009.
- Richards J, et al. Novel methods of immunogenic antigen selection for serological diagnosis of Parelaphostrongylus tenuis infection. Sci Rep. 2023;13:10989.
- Ogunremi O, et al. Serological diagnosis of Parelaphostrongylus tenuis infection in white-tailed deer and identification of a potentially unique parasite antigen. J Parasitol. 1999;85(1):122-7.














