Only about one out of three conceptions leads to birth.1 Approximately a third of embryos fail to attach to the uterus, while another third is lost after the embryo implants.
“Human reproduction is quite inefficient, and we can consider implantation [as] the bottleneck,” said Amélie Godeau, a biophysicist at the Institute for Bioengineering of Catalonia, in a press release video.
Researchers didn’t have a good way to study this process in the lab—until now. Recently, Godeau and her team developed a synthetic platform to model human embryonic development during implantation and beyond.2 Their work, published in Science Advances, allowed the researchers to visualize human embryo implantation live for the first time. This advance may one day help solve issues associated with fertility and pregnancy losses.
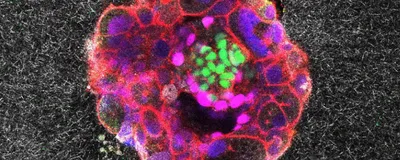
Godeau's team developed two systems, one 2D and the other 3D, to mimic different stages of embryo implantation. To simulate the extracellular environment that human embryos encounter while attaching to the womb, both platforms consisted of collagen, which is abundant in the uterus, and other proteins that are critical in early development. The researchers ensured that implanted embryos were developing properly in their synthetic womb systems by staining for standard marker proteins such as OCT4, GATA6, and CK7.
Using a high-resolution microscope, the team made time-lapse movies which showed the embryo's interaction with the synthetic matrix around it. The researchers also quantified how the embryo displaced its surrounding matrix, capturing the biomechanical dynamics of this contact.
“Our system allows the embryo to implant, and this allows us to study the development of the human embryo beyond the implantation stage,” Samuel Ojosnegros, a bioengineer at the Institute for Bioengineering of Catalonia and senior author of the work, said in the video. Human embryo implantation typically takes place around five days after fertilization, and using their platforms, the researchers could follow embryonic development for up to six days following implantation.

Samuel Ojosnegros, Anna Seriola and Amélie Godeau (from left to right), who are researchers at the Institute for Bioengineering of Catalonia, recently developed a platform that allowed researchers to visualize and quantify human embryo implantation for the first time.
Institute for Bioengineering of Catalonia (IBEC)
Godeau and Ojosnegros’s team investigated both mouse and human embryos in their platforms to see how implantation compared between the two organisms. The researchers found that both human and mouse embryos exerted mechanical forces via integrins, which are transmembrane adhesion proteins, to embed themselves onto the matrix. However, mouse embryos only invaded the matrix partially; once attached, they formed outgrowths that expanded superficially on the matrix. In contrast, human embryos didn’t form outgrowths; they completely penetrated the matrix, which eventually enveloped the embryos once they embedded themselves.
The study’s findings advanced scientists’ understanding of a limiting step in human reproduction. With robust models of human embryo implantation, researchers may someday be able to understand why this process fails so frequently and help individuals who struggle with infertility and miscarriages. In the future, Godeau, Ojosnegros, and their team would like to know how different parameters, such as extracellular matrix stiffness and embryo invasion depth, influence the mechanics of implantation.
- Larsen EC, et al. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154.
- Godeau, et al. Traction force and mechanosensitivity mediate species-specific implantation patterns in human and mouse embryos. Sci Adv. 2025.