Urinary tract infections (UTIs) are one of the most common bacterial infections worldwide, affecting hundreds of millions of people each year. A bloom of pathogenic bacteria in the urethra and bladder can cause tell-tale symptoms of the disease, such as abdominal pain, a burning sensation while urinating, and an increased urge to urinate. Between 50 and 60 percent of adult women will experience at least one UTI in their life.1 Although rare, the bacteria can creep up the tubes that carry urine to the bladder—the ureters—and infect the kidneys, leading to a serious condition called pyelonephritis, which can lead to kidney failure if left untreated. Less than three percent of uncomplicated UTIs progress to kidney disease.2 This begs the question: How does the body prevent bacterial invasion of the kidneys?
When pathogens like bacteria enter the body, neutrophils are the immune system’s first responders. Scientists have previously shown that inhibiting neutrophil recruitment results in more severe UTIs in rodents.3 Neutrophils kill microbes by engulfing them, but a flurry of recent reports show that these immune cells employ another tactic: they expel their DNA, creating large, sticky webs— neutrophil extracellular traps (NETs)—that entrap microbes.4
Now, for the first time, researchers from the University of Cambridge have observed NETs in human urine, offering the unique opportunity to explore the role of this immune mechanism in fighting urinary infections and preventing pyelonephritis. “Every individual we looked at, we could find some evidence of NETs in their urine, despite the patient not having any infection at all,” said Andrew Stewart, a nephrologist at Addenbrooke’s Hospital Cambridge and coauthor of the study. Their findings, published in Science Translational Medicine, could lead to the development of improved therapeutics to treat urinary infections of varying complexities.5
Stewart wanted to design a non-invasive way to diagnose kidney disease. To determine the extent of organ damage, healthcare professionals often need to perform a biopsy. Instead, Stewart wanted to get the same information by analyzing a patient’s urine, which contains proteins originating from various tissues in the urinary system. “We started with [urine from] healthy people and found that it was contaminated by something unusual,” he said. With the help of mass spectrometry, he observed a bunch of unknown proteins that differed from the known proteome of the urinary tract. Stewart and his team hypothesized that the mystery proteins were interacting with uromodulin (UMOD), an abundant urinary protein associated with a reduced frequency of UTIs in humans.6 They scoured databases for known UMOD binding partners, compared the proteins to their data, and discovered that the proteins in their samples were neutrophil and histone proteins.
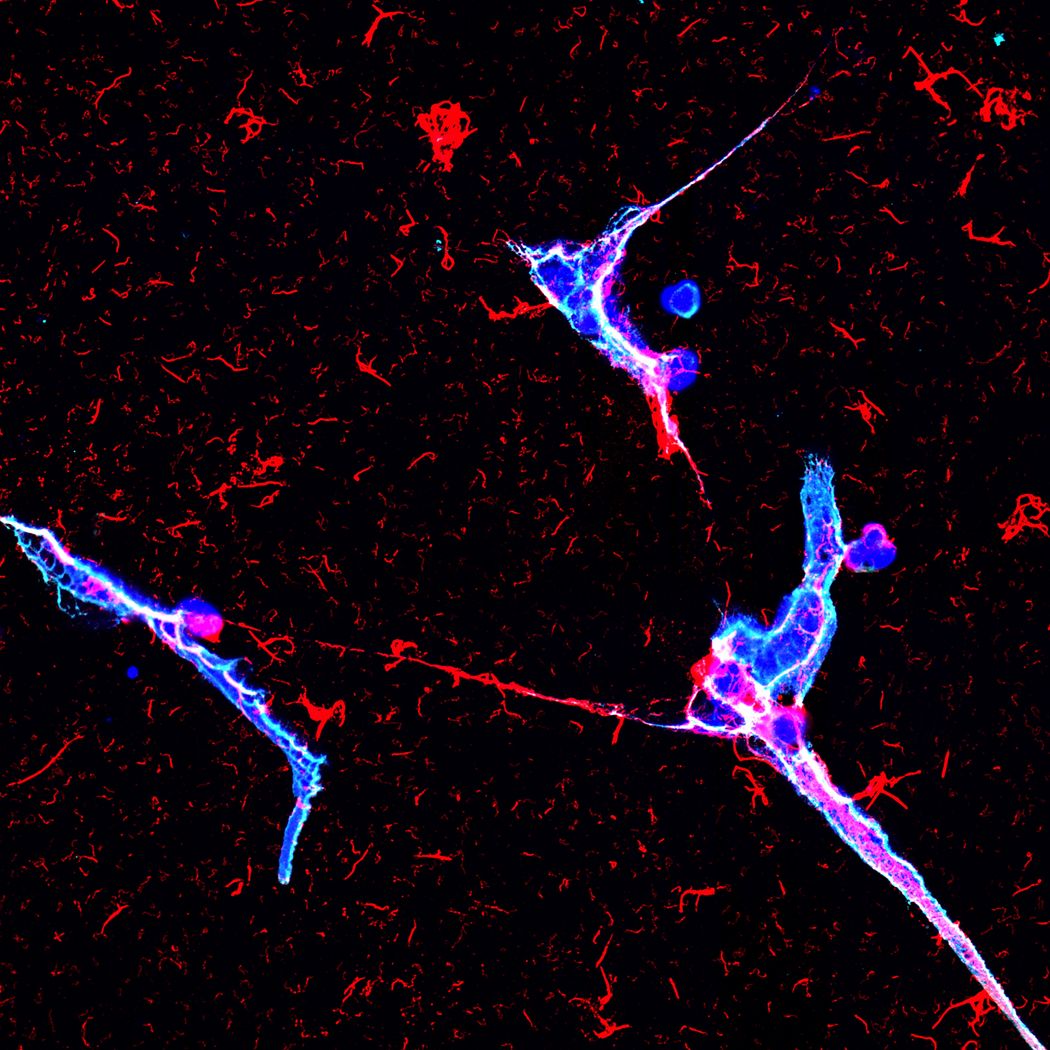
For the first time, scientists have shown the formation of NETs (blue) in healthy and infected human urine. To prevent infection of the kidney, neutrophils trap bacteria (pink) using DNA nets.
Andy Stewart, Clatworthy lab
This was in sharp contrast to the widely accepted assumption that neutrophils are rare or absent in healthy urine. However, when Stewart observed healthy human urine under the microscope, not only did he see neutrophils, but found that he could induce them to extrude their DNA and form expansive NETs decorated with UMOD. “We started off looking for something completely different and found these defensive webs of neutrophil traps in the urine,” Stewart said. “When we first saw the NETs in the urine, we went, ‘wow, this is something.’”
Next, the team wanted to know what NETs were doing in the presence of pathogens. When they induced a UTI in immunocompromised mice, they observed UMOD-coated NETs entrapping large numbers of bacteria in their bladders. Chemically inhibiting NETosis, the process of formation of NETs, led to an increase in bacteria in the kidneys. They also noticed an abundance of neutrophils in the kidneys in the presence of the NET inhibitor, but very few of them formed NETs. Blocking NETosis also diminished the aggregation of UMOD in the kidneys, as compared to mice that had pyelonephritis under normal conditions, suggesting that NETs and UMOD work cohesively to mount an antibacterial response. Stewart and his team teased apart their roles further and observed that NETs and UMOD alone were inefficient at curtailing the infection, while a combination of the two was the most potent at killing bacteria.
“If you didn't have these defense mechanisms, you'd probably have hundreds of urinary tract infections a year,” Stewart said. Despite the immune sentinels in urine, some individuals, especially females, are more susceptible to UTIs and recurring infections. Curious if genetic variants that affect NETosis also influenced the tendency to acquire a urinary infection, the team focused on the enzyme peptidyl arginine deiminase type 4 (PADI4), which is key to DNA ejection from cells. Using published data from biobanks, the team identified three single nucleotide polymorphisms in the PADI4 gene that were associated with a reduced risk of UTIs, but an increased risk of rheumatoid arthritis, suggesting a trade-off between infection susceptibility and autoimmunity.
Early detection and treatment of UTIs is another factor that is crucial for preventing pyelonephritis. One of the most commonly used tests for diagnosing UTIs is the urine dipstick test. A positive dipstick test indicates that the urine has neutrophils, and therefore pathogens. However, Stewart and his team observed that the test turned positive only in the presence of neutrophils undergoing NETosis, not intact cells, suggesting that patients who fail to test positive might be at greater risk of a kidney infection.
“This gives us a new understanding of the assay and how it may be less reliable in patients that are not able to mount this NETosis response,” said Glenn Werneburg, a urologist at Cleveland Clinic Foundation who was not involved in the study.
Stewart is interested in determining how neutrophils enter the urine, what triggers them to form NETs in different environments, and the signals that make them stop releasing their DNA. “One thing that's consistent is that size is quite important. If a microbe is really big, like fungi, NETs tend to form more often. If the microbe is small, the neutrophil might try to eat it,” he said. “Clearly, the neutrophil is making decisions about what it's going to do.” Stewart aims to understand the underpinnings of these decisions better, to be able to intervene when the process goes awry. On the flip side, Stuart speculates that excessive NETs could potentially cause problems, such as chronic cystitis. “NETosis has two edges to it. Turning it up and down may be helpful in different situations,” he said.
- Yang X, et al. Disease burden and long-term trends of urinary tract infections: A worldwide report.Front Public Health. 2022;10:888205.
- Johnson JR, Russo TA. Acute pyelonephritis in adults.N Engl J Med. 2018;378(1):48-59.
- Haraoka M, et al. Neutrophil recruitment and resistance to urinary tract infection.J Infect Dis. 1999;180(4):1220-1229
- Brinkmann V, et al. Neutrophil extracellular traps kill bacteria.Science. 2004;303(5663):1532-1535.
- Stewart AP, et al. Neutrophil extracellular traps protect the kidney from ascending infection and are required for a positive leukocyte dipstick test.Sci Transl Med. 2024;16(766):eadh5090.
- Lhotta K. Uromodulin and chronic kidney disease.Kidney Blood Press Res. 2010;33(5):393–398.
















