In the muddy waters of Mexican lakes, birds prey on axolotls by clamping their sharp teeth around the salamanders’ limbs and snapping them off. But, unlike humans who can’t regrow missing limbs, axolotls are masters of regeneration. The amputation site gradually grows a bud that eventually develops into a perfect replica of the original limb, complete with the thumb and pinky on the correct sides.
How does the axolotl’s body know to regenerate a limb at the severed site and not a tail or another tissue? This is in part due to positional memory—the spatial identity assigned to adult cells during embryonic development—that cells carry and use to restore the correct organ after an injury.1 “How the adult cells prior to amputation retain the information that the posterior and anterior cells are different was not known,” said Elly Tanaka, a regenerative biologist at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences.
Now, Tanaka and her team identified the signals that instruct cells in axolotl limbs to regrow tissues that match what was missing.2 Their results, published in Nature, reveal key molecular players in organ regeneration, with implications for regenerative medicine and tissue engineering.
“[The results] give us some glimmer of the kinds of instructions that are likely to be more effective at programming cells to make a perfect limb,” said Jessica Whited, a regenerative biologist at Harvard University, who was not associated with the study. “This is the type of really detailed and robust study that was needed to really understand how it's happening.” She added, “[This] is a basic biology paper at a time when a lot of basic biology has been under fire, especially here in the United States.”
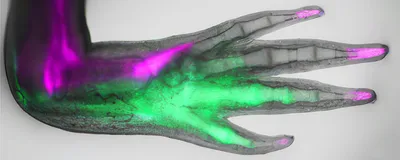
Previously, Tanaka and her team found that stem cells near the thumb on the anterior side of regenerating axolotl arms secreted fibroblast growth factor 8, while posterior stem cells near the pinky expressed Sonic hedgehog (Shh), a protein involved in embryonic development.3 This signaling loop informs patterning in the regenerating arm.
In the present study, the researchers wanted to better understand the molecular cues involved in this regeneration signaling system, focusing particularly on the regulation of Shh expression in the posterior side of the limb. They compared the transcriptional profiles of anterior and posterior cells in intact axolotl arms and observed hundreds of genes expressed differently. Among these, one stood out: Posterior cells had significantly upregulated expression of the gene called heart and neural crest derivatives expressed 2 (Hand2), which is known to induce Shh in mice and zebrafish.4,5 “When [we] saw the name of this gene, it really sent tingles up our spine,” recalled Leo Otsuki, a regenerative biologist in Tanaka’s lab. Prior work has shown that Hand2 is important for embryonic limb development.
When the researchers cut off the axolotls’ arms, Hand2 expression shot up, returning to baseline once the limb had regenerated. By lineage tracing cells that expressed the gene during embryonic development, the team found that these cells persisted in the posterior side of the arm into adulthood and were part of digits that regenerated after injury. Hand2-lineage cells also expressed Shh during regeneration.
To investigate whether Hand2 encoded posterior positional memory, Tanaka and her team expressed the gene in the anterior side of the limb, where it is not usually expressed. This resulted in a new limb growing from that location, which is consistent with previous reports of ectopic limb development because of anterior-posterior discontinuity.6 Hand2 expression in these anterior cells “posteriorized” them; they now expressed genes associated with a posterior identity.
The researchers next examined the plasticity of cell identities. They injected the anterior cells of one animal into the posterior side of another and amputated the arm after two weeks. During regeneration, the transplanted anterior cells expressed posterior cell markers, indicating a posteriorized identity.
Tanaka and her team hypothesized that the exposure of transplanted anterior cells to endogenous posterior cell-secreted Shh may have posteriorized them. Inhibiting Shh signaling during regeneration prevented Hand2 upregulation and blocked posteriorization of transplanted anterior cells. Conversely, treating anterior cells to an Shh activator during regeneration increased Hand2 expression, giving them a posterior identity, which confirmed that Shh signaling is sufficient to induce Hand2 expression and posteriorize positional memory. Overall, the results revealed a positive feedback loop between Hand2 and Shh in encoding posterior memory.
“[The results] put a defined face or molecule behind positional memory,” said Tanaka.
Otsuki added that the findings “provide a focus for looking at nonregenerative animals to ask which part of that process is working or not working.” He continued, “This gene is also in humans, so maybe our research can someday contribute towards a human understanding as well.”
Whited believes this study provides a better understanding of limb regeneration, with future applications in tissue engineering and regenerative medicine. For example, researchers could give progenitor cells in vitro specific instructions on how to grow a limb.7
However, she noted, “Limbs have more than one axis.” So future work can focus on integrating these results with molecules participating in dorsal-ventral and proximal-distal polarities.
Otsuki agreed. “We're making good progress on working on each axis one at a time, but how [do we] put all the coordinates together and rebuild all the 3D parts?” he asked. “That's going to be a challenge for an interface between biology and engineering.”
- Otsuki L, Tanaka EM. Positional memory in vertebrate regeneration: A century's insights from the salamander limb. Cold Spring Harb Perspect Biol. 2022;14(6):a040899.
- Otsuki L, et al. Molecular basis of positional memory in limb regeneration. Nature. 2025;642: 730–738
- Nacu E, et al. FGF8 and SHH substitute for anterior-posterior tissue interactions to induce limb regeneration. Nature. 2016;533(7603):407-410.
- Charité J, et al. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127(11):2461-2470.
- Yelon D, et al. The bHLH transcription factor Hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127(12):2573-2582.
- Endo T, et al. A stepwise model system for limb regeneration. Dev Biol. 2004;270(1):135-145.
- Chen Y, et al. Generation of iPSC-derived limb progenitor-like cells for stimulating phalange regeneration in the adult mouse. Cell Discov. 2017;3:17046.