Researchers have identified the function of an obscure but large family of proteins whose function in cellular immune responses had been unknown. 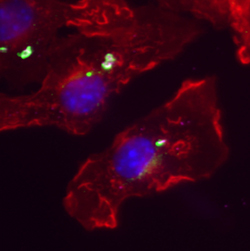 |
Fluorescent micrograph of Gbp1 (green) targeting mycobacteria (magenta rods) in interferon-activated macrophages (red, actin staining;
blue, nuclear staining)
Image courtesy of John MacMicking |
Microbiologist John MacMicking shows how specialized proteins
battle bacteria inside immune cells.
Video courtesy of Science and John MacMicking
**__Related stories:__***linkurl:Cellular chaos fights infection;http://www.the-scientist.com/news/display/57982/
[10th February 2011] *linkurl:RNA Arms Race;http://www.the-scientist.com/2010/8/1/51/1/
[1st August 2010] *linkurl:Antiviral response promotes bacterial infection;http://www.the-scientist.com/news/display/25036/
[10th October 2006]




















