The 2025 Nobel Prize for Physiology or Medicine was awarded to Shimon Sakaguchi, an immunologist at Osaka University; Fred Ramsdell, an immunologist at Sonoma Biotherapeutics; and Mary Brunkow, a molecular biologist at the Institute for Systems Biology, for their work in identifying and defining regulatory T cells (Tregs). The immune system acts like a police force for the body, identifying external and internal threats against the body’s cells. If the patrol officers of this corps are cell-killing actors like effector T cells, then regulatory T cells are the force’s internal affairs division.1
The Origin of Regulatory T Cells
The regulatory division of the immune system is vital for its overall function. Immune T cells originate in the bone marrow and then travel to the thymus—a kind of biological police academy.2 Here, the body assesses trainee T cells to ensure they bind to the correct types of receptors, which will enable them to respond appropriately to threats in the rest of the body.
During this training period, a process called central tolerance ensures that T and B immune cells with receptors that are too firmly aimed at the body’s own structures are destroyed before they can escape into the periphery and cause havoc. However, this process is not perfect, and sometimes rogue immune cells break free, leading to autoimmune conditions such as rheumatoid arthritis or multiple sclerosis.3 This is where Tregs activate their sirens and begin pursuit.
Researchers only fully characterized Tregs at the start of the 21st century, despite their essential role in the immune system. That’s because these cells are rare and unusually variable. Immunologists organize Tregs into three subdivisions, based on where they emerge in the body.
Three Types of Regulatory T Cells
A candidate for an internal affairs position needs to be willing to hunt down their rogue colleagues. During immune police academy, some developing cells will respond more strongly to self-antigens than others, but not so strongly that they are marked out as threats to the body.4 These will ultimately become thymus-derived Tregs (tTregs). Immunologists characterize tTregs by the expression of specific chemical markers on their surface and within their cell body. When looking for tTregs, researchers select cells that are positive for a surface marker called cluster of differentiation (CD)4, have a high level of another marker, CD25, and do not display a marker called CD127. Importantly, these cells will also express a transcription factor called FOXP3.
Outside the thymus, on the mean streets of the body, T cells patrol, looking for threats. These cells will have a complementary antigen target to which they respond strongly. Before they encounter that specific threat, they exist in a naïve state that is undifferentiated and flexible.5 Some of these cells, when encountering chemical signals outside the thymus will adopt Treg-like characteristics. These cells are called peripheral Tregs (pTregs). These are like rank-and-file officers who are tapped on the shoulder to become internal affairs specialists. There are not currently any markers that reliably distinguish tTregs from pTregs in humans.6
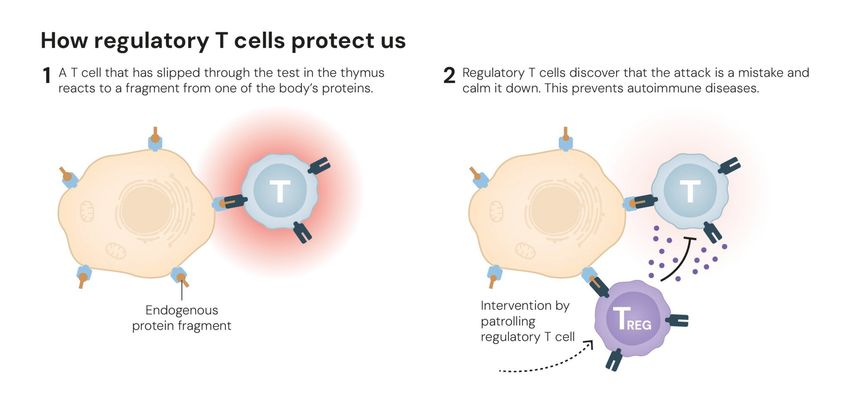
Regulatory T cells identify and remove T cells that could harm the body, preventing autoimmunity.
© The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Finally, outside the body, bathing naïve T cells in specific concentrations of chemicals transforms them into Treg-like cells. These induced Tregs (iTregs) can be distinguished from their in vivo counterparts through analysis of a particular region of the FOXP3 gene, known as the Treg-specific demethylated region (TSDR). This region is demethylated in tTregs and pTregs, but methylated in iTregs. The functional effects of this difference aren’t yet apparent.
While FOXP3 is considered the “master regulator” of all Tregs’ suppressive abilities, cells that aren't suppressive can also express it. Identifying Tregs’ unique combination of surface and internal markers is essential to isolating these cells. One factor that complicates the identification of Tregs is that they can also go undercover by adopting markers usually present on their target cells. This can help them more effectively reach and suppress these cells.
How Regulatory T Cells Suppress Autoimmunity
When Tregs of any shade catch up with rogue T cells, they have an arsenal of methods through which they suppress the rogue T cells’ autoimmunity.7 First, they can release chemicals called cytokines that quench the target cells. They can also bring to bear enzymes called granzymes that physically break apart rogue cells. Tregs can also use metabolic trickery in their pursuit. They can rapidly consume local supplies of a vital chemical called interleukin (IL)-2, which starves their target and directs it to self-destruct via a process called apoptosis. Finally, they can target other cell types. Antigen-presenting cells play a key role in autoimmunity by exposing rogue T cells to self-antigens. Tregs can suppress this process by producing a kind of chemical "grabber arm" that physically removes the presented self-antigen.8
Tregs’ chemical flexibility and the complex web of markers required to isolate them delayed their discovery for many years. The awarding of the 2025 Nobel Prize to Sakaguchi, Ramsdell, and Brunkow reflects the significance of these cells’ contribution to the immune system.
- Vignali DAA, et al. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532.
- Meng X, et al. Immunological mechanisms of tolerance: Central, peripheral and the role of T and B cells. Asia Pac Allergy. 2023;13(4):175–186.
- Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665–673.
- Workman CJ, et al. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66(16):2603–2622.
- Berard M, Tough DF. Qualitative differences between naïve and memory T cells. Immunol. 2002;106(2):127–138.
- Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: Similarities and differences. Immunol Rev. 2014;259(1):88–102.
- Gouirand V, et al. Regulatory T Cells and Inflammatory mediators in autoimmune disease. J Invest Dermatol. 2022;142(3, Part B):774–780.
- Huang Q, et al. Suppression of human dendritic cells by regulatory T cells. Bio Protoc. 2021;11(21):e4217.














