Skeletal muscle has remarkable regenerative capacity thanks to satellite cells, a special stem cell type that self-renews and creates progenitor cells, which fuse and engraft into muscle fibers.1 These characteristics contribute to the therapeutic potential of satellite cells in degenerative and disease states that impede muscle regeneration. However, when scientists isolate satellite cells from the skeletal muscle niche for 2D cell culture, they begin to fight a losing battle against stem cell differentiation. “They basically spontaneously differentiate into myoblasts. They are no longer muscle stem cells, they are what we call committed muscle progenitors,” said Lee Rubin, a neuroscientist at Harvard University who studies central nervous system aging and skeletal muscle disorders. “Myoblasts certainly can make muscle, but what they cannot do is reoccupy their niche … This is a big problem for the muscle field.”

Lee Rubin, a neuroscientist at Harvard University, uses organoids to study central nervous system aging and skeletal muscle disorders.
Kris Snibbe, Harvard University
In work published in Nature Biotechnology, Rubin and his research group turned to 3D cell culture to take on the problem of generating sufficient satellite cells for regenerative therapies.2 Spearheaded by cell biologist Feodor Price, lead author of the study and principal scientist in Rubin’s laboratory, the researchers created progenitor-derived skeletal muscle organoid (SkMO) models to recapitulate the satellite cell niche in vitro. Within this SkMO niche, they found, isolated, and expanded functional satellite-like cells, which they named in vitro-derived satellite cells (idSCs). These new stem cells were distinct from naturally occurring satellite cells and capable of repairing damaged or diseased muscle in mouse models of degeneration.
Researchers typically create organoid models for other biological systems with pluripotent stem cells, but the Harvard team began with readily accessible myoblasts, which satellite cells differentiate into in vivo and in 2D culture. Under specific 3D culture conditions, the myoblasts self-assembled into SkMO and differentiated further into other mature muscle cell types. Price also identified cells that lacked the myoblast marker myoblast determination protein 1 (MyoD) but expressed the satellite cell marker paired box 7 (Pax7). “What he found was that, in the niche created in the organoid, satellite cells that were not there to start with appeared. In other words, that the myoblasts had de-differentiated into more of a satellite-like cell,” Rubin explained.
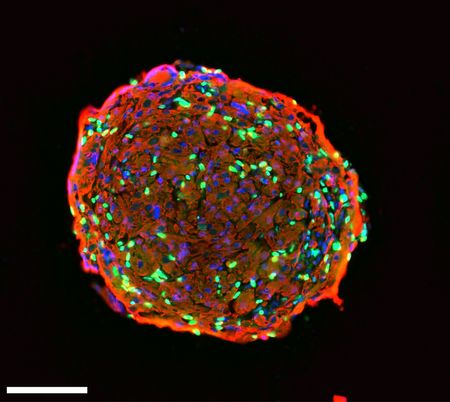
Feodor Price grew human skeletal muscle organoids from myoblasts, which differentiated into mature muscle cells and de-differentiated into expandable and novel satellite-like cells. In this microscopy image of a 15-day old skeletal muscle organoid, taken with a Nikon Eclipse Ti, cell nuclei are stained in blue, myosin heavy chain in red, and Pax7 in green (100μm scale bar).
The Rubin Lab
These newly identified idSCs were genetically and epigenetically distinct from satellite cells and expandable in 3D culture, which allowed the scientists to generate millions of cells and overcome the roadblock of in vitro satellite cell production. “It's an exciting paper because the culture of muscle stem cells results in their loss of engrafting potential,” said Shahragim Tajbakhsh, a stem cell biologist at the Pasteur Institute who investigates skeletal muscle development and regeneration, and who was not involved in the study. “The major output of this paper is that they've come up with a protocol that circumvents that.”
In addition to producing large numbers of satellite-like cells, Price and Rubin transplanted the cells into mouse models with damaged muscle and demonstrated that idSC are functionally better than myoblasts at repopulating the stem cell niche, and comparable to if not better than satellite cells freshly isolated from muscle. “They have come up with a protocol that is not completely matching the in vivo state but has a reasonable and very exciting signature that is matching that in vivo state,” said Tajbakhsh. “When they compare it to myoblasts and when they compare it to bona fide in vivo derived muscle stem cells, they've probably reached the closest protocol to the in vivo state that I have seen to date.”
- Briggs D, Morgan JE. Recent progress in satellite cell/myoblast engraftment – relevance for therapy. FEBS J. 2013;280(17):4281-4293.
- Price FD, et al. Organoid culture promotes dedifferentiation of mouse myoblasts into stem cells capable of complete muscle regeneration. Nat Biotechnol. September 2024. Online ahead of print.














