T
racking neuronal fibers in the central nervous system is critical for understanding the organization and pathophysiology of the brain. Neuronal connections between functional areas of the brain have been historically conducted using invasive techniques in experimental animals which were often terminal procedures. The recent advent of modern technologies has advanced neuroanatomical applications and ushered in a new era of tracing neuronal fiber tracts in living brains.1 This article examines several neuroanatomical tracing techniques and their associated nuances with each system.
Mapping initial trails: Staining Protocols
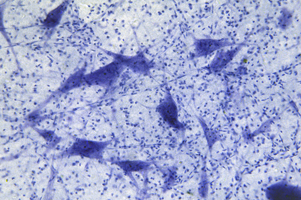 The earliest examination of brain anatomy involved staining protocols to label neurons, myelin, and nuclei. This includes initial renditions of silver stains developed by Camilo Golgi which was further refined by Ramon y Cajal to visualize neurons using light microscopy. Other standard staining methods such as Hematoxylin and Eosin could differentiate axons and dendrites from astrocytes, oligodendrocytes and glial cells based on a cell’s acidophilic and basophilic properties. Dyes such as Luxol-fast blue (myelin), Nissl (neuronal bodies), and Holzer (astrocytes) began to be regularly incorporated in research laboratories studying neurons. These were complemented with specialized stains targeted for abnormal neuropathological structures including Gallyas-Braak (tau deposits) and Methenamine Silver (β-amyloid plaques).
The earliest examination of brain anatomy involved staining protocols to label neurons, myelin, and nuclei. This includes initial renditions of silver stains developed by Camilo Golgi which was further refined by Ramon y Cajal to visualize neurons using light microscopy. Other standard staining methods such as Hematoxylin and Eosin could differentiate axons and dendrites from astrocytes, oligodendrocytes and glial cells based on a cell’s acidophilic and basophilic properties. Dyes such as Luxol-fast blue (myelin), Nissl (neuronal bodies), and Holzer (astrocytes) began to be regularly incorporated in research laboratories studying neurons. These were complemented with specialized stains targeted for abnormal neuropathological structures including Gallyas-Braak (tau deposits) and Methenamine Silver (β-amyloid plaques).
Keeping Track: The rise of Neuro-tracers
Tracing neurons is achieved through monitoring axonal flow between synapses and neuronal cell bodies. Lipophilic fluorescent neuro-tracers such as DiI (1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindocarbocyanine Perchlorate) allow for anterograde and retrograde axoplasmic flow monitoring.2 Other retrograde neuro-tracers ...














