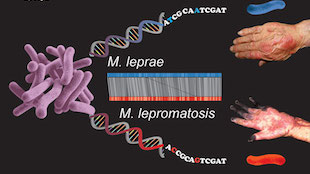
 IMAGE COURTESY OF LUCIO VERA-CABRERAUntil recently, researchers believed that leprosy was caused by a single species of bacterium, Mycobacterium leprae. To the surprise of the leprosy research community, in 2008, a pathologist discovered an entirely new leprosy-causing species in the liver of a deceased man from Mexico. A new full genome sequence of the organism, M. lepromatosis, shows that despite having diverged 13.9 million years ago, the two leprosy-causing species are nevertheless remarkably similar, genetically. A team led by researchers from École Polytechnique Fédérale de Lausanne in Switzerland described the M. lepromatosis genome in PNAS this week (March 23).
IMAGE COURTESY OF LUCIO VERA-CABRERAUntil recently, researchers believed that leprosy was caused by a single species of bacterium, Mycobacterium leprae. To the surprise of the leprosy research community, in 2008, a pathologist discovered an entirely new leprosy-causing species in the liver of a deceased man from Mexico. A new full genome sequence of the organism, M. lepromatosis, shows that despite having diverged 13.9 million years ago, the two leprosy-causing species are nevertheless remarkably similar, genetically. A team led by researchers from École Polytechnique Fédérale de Lausanne in Switzerland described the M. lepromatosis genome in PNAS this week (March 23).
“It appears, to me, to be a landmark paper which really provides a bounty of insight into this newly recognized and little-understood organism,” said Richard Truman, chief of the laboratory research branch at the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. Truman, who was not involved in the research, said that M. lepromatosis was originally only characterized using a few snippets of DNA, causing some skepticism in the community about whether it was truly a new species. The sequence, he added, ends any lingering confusion.
“It’s a really beautiful study,” said Xiang-Yang Han, the pathologist who discovered M. lepromatosis. Han, who analyzes patient samples at the MD Anderson Cancer Center in Houston, Texas, was not involved in the present study but said he ...















