During brain development, an excess of neurons and neurites, the cellular projections that will eventually develop into axons and dendrites, are produced. These are later scaled back either by pruning of unneeded projections or apoptosis of whole cells. Aberrations in this process have been associated with neurological conditions like autism spectrum disorder and epilepsy.1,2 Better understanding of the mechanisms underlying this remodeling could provide new insights into these disorders or inform novel therapeutic strategies.
Research on remodeling in flies has largely focused on a few different types of neurons; in these populations, the steroid hormone ecdysone is a key driver of this developmental process.3 However, many other types of neurons remain underexplored, leaving the possibility of novel regulatory mechanisms open. “We still don't have a really good grasp on all the genetic and molecular mechanisms that can drive these processes,” said Katherine Lehmann, a neurobiologist who is currently a science policy fellow at the National Institutes of Neurological Disorders and Stroke.
In a study published in the Journal of Cell Biology, Lehmann and her colleagues at the Oregon Health and Science University (OHSU) identified a population of neurons that underwent remodeling through distinct mechanisms compared to previously studied cells.4 The findings help scientists better understand this process in brain development and neurological disorders.
When Lehmann began her doctoral research in Marc Freeman’s group at OHSU, her first goal was to identify neuron populations that underwent remodeling by distinct mechanisms. To do so, Lehmann screened 5,500 fly lines for gene expression in the ventral nerve cord, similar to the spinal cord in vertebrates, in the stage just prior to metamorphosis. By studying how gene expression patterns changed over time, she identified a population of motor neurons called Beat-Va neurons, that had not previously been studied in the context of developmental remodeling.
Within the Beat-Va neurons, the researchers observed two populations of these cells: lateral and medial (Beat-VaL and Beat-VaM, respectively). Imaging the cells throughout the early stages of metamorphosis revealed that populations of Beat-VaL neurons in certain abdominal regions underwent apoptosis but remained intact in other regions. Beat-VaM neurons avoided cell death in multiple areas of the body, but many of their neurites were pruned away.
To determine if ecdysone controlled either of these remodeling processes, the researchers blocked the hormone signaling in the cells. Beat-VaM remodeling was unaffected, but this blockade prevented apoptosis in Beat-VaL cells.
To explore the body segment-specific cell death of the Beat-VaL population, the team investigated the expression of the gene Abdominal-B, which defines the boundary between anterior and posterior cells. The expression of Abdominal-B in Beat-VaL neurons corresponded with the segments that experienced loss of these cells during maturation, and knockdown of this gene led to reduced Beat-VaL cell death.
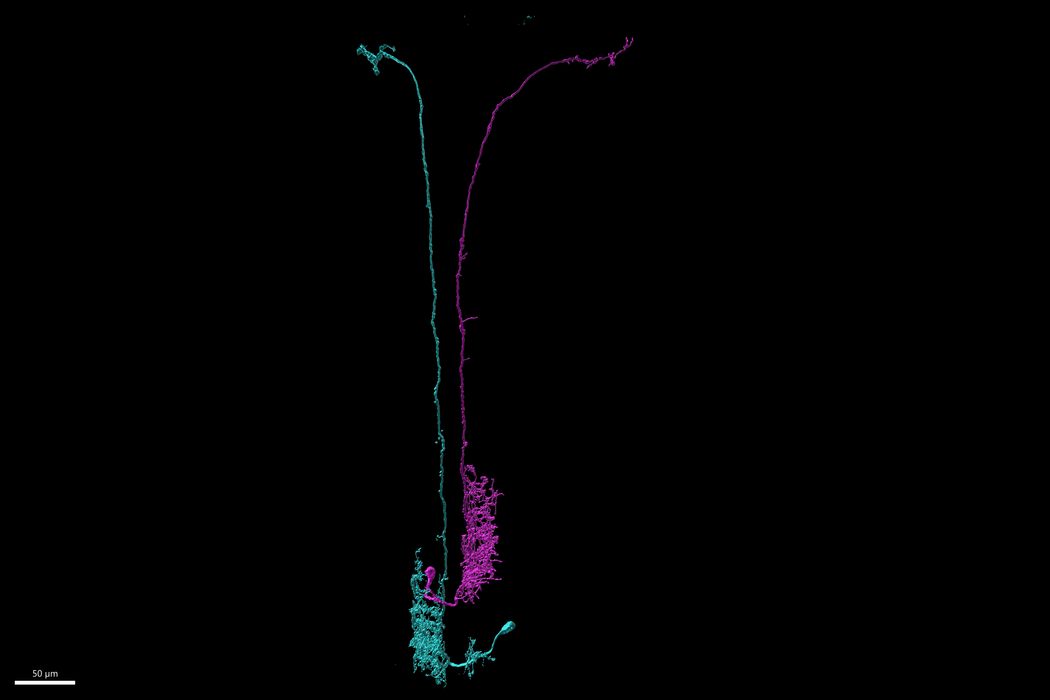
Researchers identified medial Beat-Va neurons underwent a pruning mechanism that was independent of their ecdysone signaling.
Katy Lehmann
Next, they explored remodeling in the Beat-VaM neurons. “The surprising thing is that it is ecdysone-independent,” said Jean-Maurice Dura, a neuroscientist at the National Center of Scientific Research who was not involved in the study. “In the [fly brain], if you block the ecdysone signaling within [other types of neurons], you block everything.”
To confirm that these Beat-VaM neurons did not respond to ecdysone signaling, Lehmann tried various approaches to inhibit the activity of this molecule. “The neurons didn't care. They just kept remodeling,” she recalled. “That sort of led us to explore sort of novel ideas of like, all right, what if it isn't a cell intrinsic mechanism?”
Although these neurons remodeled independently of ecdysone, these cells underwent neurite pruning after the release of the hormone. During this developmental period, ecdysone drives some astrocytes in the larva to become phagocytic. When the researchers blocked ecdysone signaling in astrocytes, these cells did not become phagocytes and Beat-VaM pruning was subsequently reduced.
“Everything we found that stopped remodeling also stopped astrocyte transformation, which opens up this really interesting question of like, what is going on during astrocyte transformation that is so important for these neurons to fall apart,” Lehmann said.
To further study these phagocytic cells’ role in Beat-VaM pruning, the team knocked down individual genes that corresponded to cell surface markers. They observed that while some of these resulted in the neurons fragmenting but the debris not being cleared, manipulations that prevented phagocyte transformation resulted in a loss of neuron pruning, supporting an active role of these phagocytic cells in Beat-VaM neuron remodeling.
Dura said that he is interested in seeing future exploration into how these phagocytic cells contribute to the neuron fragmentation. Madison Hupp, a graduate student in Freeman’s group and study coauthor, said that exploring this mechanism is something the group wants to follow up on.
Hupp added that their findings in the Beat-Va neurons encouraged the team to explore more neuron populations and their remodeling mechanisms. “We're finding that it's even more diverse,” she said. “It’s kind of interesting how you have kind of the same processes happening, but it's happening in so many different ways.”
- Ishizuka K, et al. Rare genetic variants in CX3CR1 and their contribution to the increased risk of schizophrenia and autism spectrum disorders. Transl Psychiatry. 2017;7(8):e1184.
- Neniskyte U, Gross CT. Errant gardeners: Glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 2017;18(11):658-670.
- Yaniv SP, Schuldiner O. A fly’s view of neuronal remodeling. Wiley Interdiscip Rev Dev Biol. 2016;5(5):618-635.
- Lehmann KS, et al. Astrocyte-dependent local neurite pruning in Beat-Va neurons. J Cell Biol. 2025;224(1):e202312043.















