 |
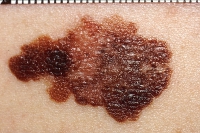
Image: National Cancer Institute |
**__Related stories:__***linkurl:Q&A: Is stem cell research misguided?;http://www.the-scientist.com/blog/display/56024/
[29th September 2009]*linkurl:Cancer's culprit;http://www.the-scientist.com/article/display/55537/
[April 2009]*linkurl:A life behind life science;http://www.the-scientist.com/article/display/54576/
[May 2008]




















