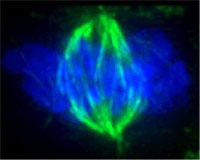
 |
spindle (green) during cell division Image: Oak Ridge Nat'l Lab, via Wikipedia |
**__Related stories:__*** linkurl:All systems go;http://www.the-scientist.com/article/display/55451/
[March 2009]*linkurl:Cell division rewinds;http://www.the-scientist.com/article/display/23551/
[June 2006]*linkurl:In cell cycle, size matters;http://www.the-scientist.com/article/display/22361/
[25th August 2004]



















