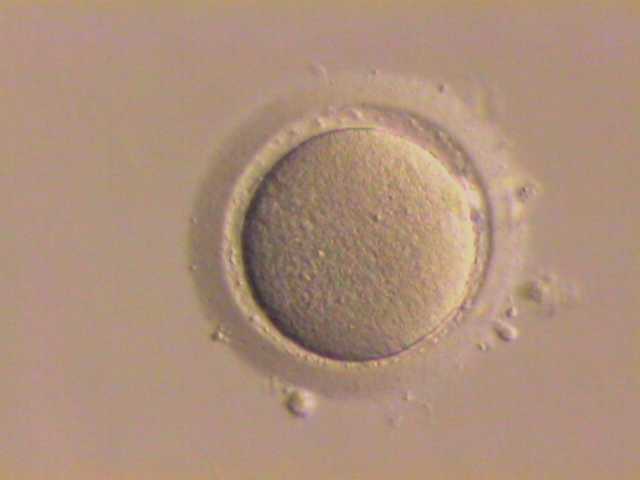
 |
Image: Wikimedia Commons |
**__Related stories:__***linkurl:New eggs from old mice?;http://www.the-scientist.com/article/display/53228/
[1st June 2007]*linkurl:No mature eggs from bone marrow;http://www.the-scientist.com/news/display/23653/
[15th June 2006]*linkurl:New oocytes from bone marrow?;http://www.the-scientist.com/article/display/22742/
[28th July 2005]




















