 Blocked Swapping
Blocked Swapping
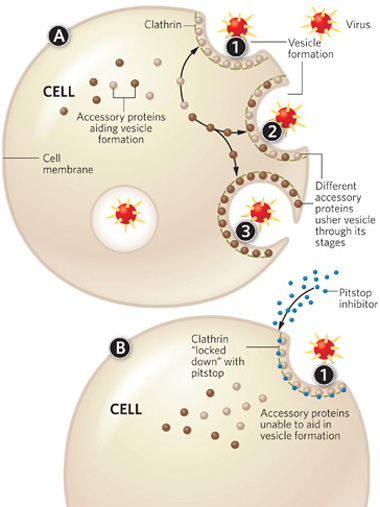
A. The clathrin lattice forms at the site of membrane invagination (1). Underneath the lattice accessory proteins come and go (2), each driving a different step in the invagination and vesicle-forming process (3). B. When a pitstop binds clathrin (1), it prevents these accessory proteins from binding to clathrin, and endocytosis stops. PRECISION GRAPHICS
Clathrin-mediated endocytosis (CME)—by which cells ingest extracellular molecules and internalize cell surface proteins—is an essential process in practically all cells. Now, Volker Haucke of Freie Universität in Berlin and colleagues have identified two small molecules, which they named “pitstops” 1 and 2, that bind to clathrin’s terminal domain and stop CME. The inhibitors not only will help researchers elucidate clathrin’s molecular mechanisms, but could also serve as the basis for designing CME-preventing drugs for a variety of clinical applications.
“There are different ways to inhibit clathrin,” such as overexpressing or mutating accessory proteins, or using RNAi to deplete clathrin, says Ludger Johannes of the Institut Curie in Paris, who was not involved in the study. “But these all have limitations,” he adds. For example, with ...


















