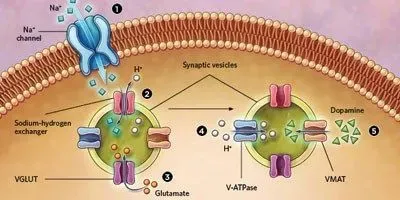
PUMPING IT UP: Researchers have found that synaptic vesicles releasing dopamine across neuronal synapses in fruit flies and mice can dynamically adjust the neurotransmitter content in response to neuronal firing. And they propose a mechanism to explain how. When the axon terminal depolarizes, sodium ions flow into the cell (1). The increased sodium ion concentration activates sodium-hydrogen exchangers in the vesicle membrane that transport one sodium ion into the vesicle for every proton out (2). This action increases the difference in electrical charge across the vesicle membrane, activating a transporter protein, VGLUT, which pumps another neurotransmitter, negatively charged glutamate, into the vesicle (3). The resulting buildup of internal negative charge triggers the pumping of more protons into the vesicle, increasing the pH gradient across the membrane (4). Finally, this drop in pH inside the vesicle triggers the loading of more dopamine into the vesicles via the VMAT protein (5).© EVAN OTO/SCIENCE SOURCE
PUMPING IT UP: Researchers have found that synaptic vesicles releasing dopamine across neuronal synapses in fruit flies and mice can dynamically adjust the neurotransmitter content in response to neuronal firing. And they propose a mechanism to explain how. When the axon terminal depolarizes, sodium ions flow into the cell (1). The increased sodium ion concentration activates sodium-hydrogen exchangers in the vesicle membrane that transport one sodium ion into the vesicle for every proton out (2). This action increases the difference in electrical charge across the vesicle membrane, activating a transporter protein, VGLUT, which pumps another neurotransmitter, negatively charged glutamate, into the vesicle (3). The resulting buildup of internal negative charge triggers the pumping of more protons into the vesicle, increasing the pH gradient across the membrane (4). Finally, this drop in pH inside the vesicle triggers the loading of more dopamine into the vesicles via the VMAT protein (5).© EVAN OTO/SCIENCE SOURCE
The paper
J.I. Aguilar, “Neuronal depolarization drives increased dopamine synaptic vesicle loading via VGLUT,” Neuron, 95:1074-88.e7, 2017.
Psychiatrist Zachary Freyberg thought he knew the basics of dopamine signaling. When a dopamine neuron fires, vesicles containing the neurotransmitter migrate to the cell membrane, where they fuse and release their cargo into the synapse, all in the course of about a millisecond. But a chance observation by Freyberg a few years ago revealed a new dimension to this critical aspect of neural communication.
At Columbia University, beginning in 2009, Freyberg had helped develop a technique to observe dopamine signaling in living Drosophila brains. The method used molecules of FFN206, Freyberg says, which “behave like dopamine, but unlike dopamine, they’re fluorescent and therefore can be readily visualized.”
He ...