To study biological processes, scientists often take snapshots of the molecules in cells, such as proteins and nucleotides, at specific points in time. In spatial biology, they can leverage molecular approaches using imaging techniques, uncovering where these molecules localize in cells and tissues. This can allow researchers to gain a more complete picture of how cells regulate processes in both space and time. In this article, learn how spatial biology has helped scientists better understand various biological phenomena, such as antibiotic resistance, which received one of this year’s Lasker Awards.

Shapiro’s research using the bacterium Caulobacter crescentus revealed important insights about spatial organization in bacterial cells, overturning the then-prevalent notion that bacteria are sacs of jumbled enzymes.
Christopher Michel
Researcher Who Uncovered Spatial Regulation of Bacterial Genes Wins Lasker Award
In September 2025, Lucy Shapiro, a painter-turned-developmental-biologist at Stanford University, received the Lasker Award for her pioneering study of bacterial gene regulation in three dimensions. In a career that spans more than five decades, Shapiro studies how spatial dynamics affects gene regulation in Caulobacter crescentus, a crescent-shaped bacterium. Along with her physicist husband, Harley McAdams, Shapiro showed the high extent to which bacterial chromosomes are spatially organized using sequencing and imaging techniques, which was a revolutionary insight in the early 2000s.
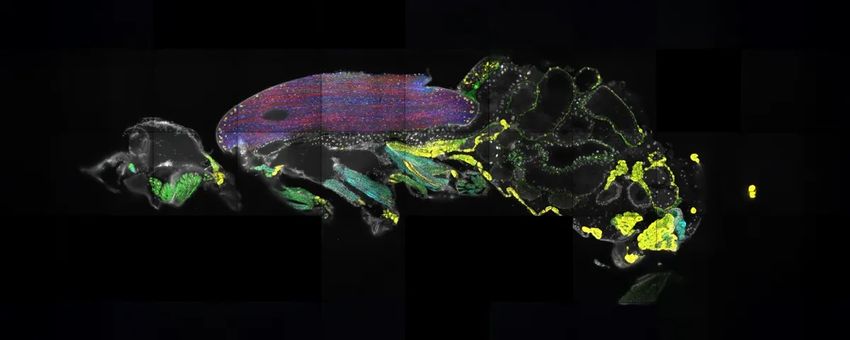
Scientists used spatial transcriptomics to locate the positions of mRNAs throughout the body of a fruit fly.
Frank Schnorrer / License CC-BY 4.0, Janssens et al, 2025, eLife
Whole-Body Spatial Transcriptomics in the Fruit Fly Reveal Novel Insights
Most information about spatial regulation is lost when researchers perform molecular experiments like RNA sequencing. Spatial transcriptomics, an approach that reveals the nature as well as the location of transcripts in cells, can bridge this gap. Recently, researchers used this method to learn the positions of 150 mRNA transcripts (100 expressed in the fly’s brain and 50 in the body) across the entire body of an adult fruit fly. They froze a fruit fly and sliced it into 10-micrometer-thick sections, then imaged the transcripts within the intact tissue sections. Their approach revealed novel, surprising insights, including how transcripts encoding titin, an abundant muscle protein, are concentrated at the edges of the flight muscle although the protein is needed across the entire tissue.
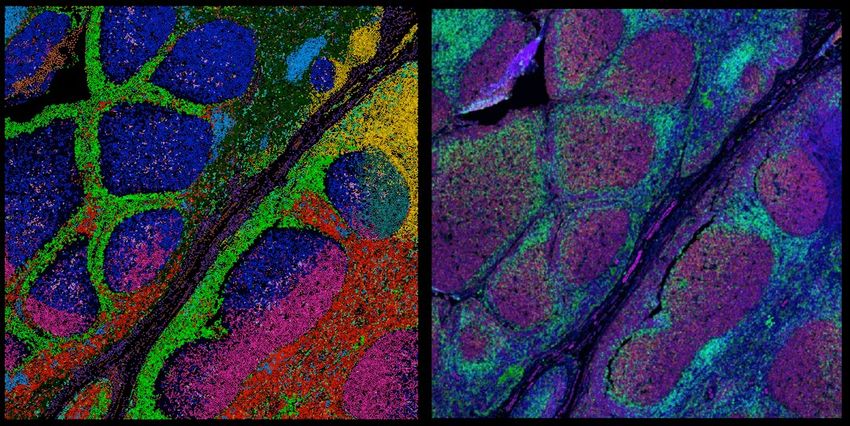
Spatial proteomics is a marriage between two fields, imaging and proteomics. It allows scientists to profile cells' and tissues' proteomes without losing information about the molecules’ native context.
Rong Fan
Deep Visual Proteomics Advances Transform Cell Biology
According to Matthias Mann, a biochemist at the Max Planck Institute of Biochemistry, spatial proteomics marries two key research fields, proteomics and microscopy. The earliest spatial proteomics methods visualize proteins in biological samples using immunofluorescent antibodies. Later developments of the approach allow researchers to combine imaging with mass spectrometry (MS), an unbiased, high-throughput approach to study proteins in cells and tissues. Most recently, Mann and his collaborator, Andreas Mund, an engineer at the University of Copenhagen, improved MS imaging further in an approach called deep visual proteomics. With this method, researchers can analyze can profile the proteomes of individual cells in a tissue section using MS, then map this information back into the tissue, capturing the full complexity of biological processes.
Deep Visual Proteomics Identify Cause and Potential Cure for a Deadly Skin Disease
Toxic epidermal necrolysis is widely considered as the only emergency in the field of dermatology. This rare condition, which is typically characterized by large areas of an affected individual’s skin blistering and peeling, is often fatal and has no cure. Recently, Thierry Nordmann, a biochemist at Max Planck Institute, performed deep visual proteomics to compare skin and immune cells in the mild and lethal forms of the disease. Working in Mann’s laboratory, Nordmann discovered a dramatic upregulation of interferon signaling in more severe forms of the disease. His team’s results revealed potential pharmacological targets for this horrifying and life-threatening condition.

Sean Bendall (left) and Thomas Montine (right) used multiplexed ion beam imaging to create a spatial proteomic atlas of postmortem human brains from both cognitively healthy people and people with Alzheimer’s disease.
Sean Bendall and Thomas Montine
Spatial Proteomics Atlas Indicates Microglia Exhaustion in Alzheimer’s Disease
Thomas Montine, a neuropathologist at Stanford University, wondered if immune cells are “consuming” the brain in Alzheimer’s disease the way that they do in tuberculosis. Recently, in a collaboration with Sean Bendall, another pathologist at Stanford University, Montine dove deep into the role of the microglia, the brain’s resident immune cells, in Alzheimer’s disease. The researchers generated a spatial proteomics atlas of the microglia using the brains of individuals with and without the neurodegenerative disease. The maps revealed a wide range of immune activation states of the microglia, and this variation was organized spatially. The researchers also observed patterns that indicate immune exhaustion in the microglia of individuals with Alzheimer’s disease. Montine hopes that his team’s findings may provide clues as to how Alzheimer’s disease pushes the microglia towards immune exhaustion and if their normal function may be restored.
Spatial Approach Helps Researchers Understand Whipworm Infections
Whipworms are intestinal parasites that have plagued modern and ancient humans, including the 5,300-year-old “Ötzi the Iceman”. To understand how whipworms infect the digestive tract, researchers like Maria Duque-Correa, an immunologist at the University of Cambridge, use mouse models and three-dimensional gut organoids. They filmed whipworms burrowing into the tissue and observed how these parasites fought with the cells that tried to get rid of them. Duque-Correa hopes that these models can help researchers develop new and better drugs to treat whipworm infections.
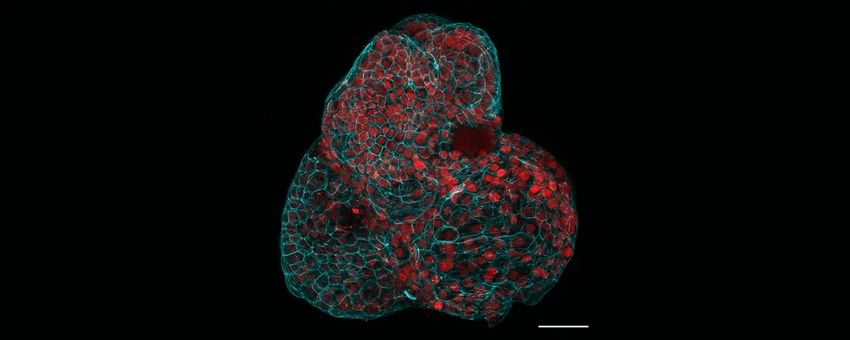
Researchers generated various human fetal organoids, including lungs, from the amniotic fluid.
Giuseppe Calà, Paolo De Coppi, Mattia Gerli
Spatial Studies on Fetal Organoids May Reveal Insights into Rare Developmental Disorder
About one-third of babies who are born with congenital diaphragmatic hernia (CDH), a rare disorder affecting lung disorder, do not survive. To study how CDH occurs and impacts organ development is limited, researchers led by Mattia Gerli, a stem cell biologist at University College London, recently generated fetal organoids using cells from the amniotic fluid, the protective yellowish liquid that surrounds a developing fetus. Gerli and his colleagues collected amniotic fluid before and after physicians perform surgical intervention to treat CDH, then compared organoids derived from the two different time points. The team observed gene expression patterns that indicate tissue maturation following surgery, indicating the organoids’ potential in helping researchers understand fetal organ development and CDH progression.