Organoids—tiny, lab-grown 3D clusters of cells—are a useful tool for studying organ development, disease, and drug responses. These miniature organs-in-a-dish are powerful, but they’re not perfect: Recreating the complex interplay of different cell types found in full-sized organs remains tricky. For example, most pancreatic organoids only contain a single cell type, which makes it difficult for researchers to fully understand how different cell types work together in a functioning pancreas.
So when Amanda Andersson-Rolf, a postdoctoral researcher in Hans Clevers’s group at the Hubrecht Institute, set out to build a better pancreatic organoid, she decided to include all three main cell types found in this organ. She would have to figure out how to co-culture acinar cells, which produce digestive enzymes, ductal cells, which transport these enzymes, and endocrine cells, which regulate blood sugar levels by releasing hormones.
Clevers’s lab previously generated pancreatic organoids from adult mice and human tissue-resident stem cells (TSCs), but these only produced ductal cells.1,2 Andersson-Rolf aimed to slowly expand their current model, trying to coax it to produce endocrine cells as well, but her attempts were unsuccessful. Since organoids derived from adult cells, such as induced pluripotent stem cells or TSCs, can be limited in the cell lineages they generate, Andersson-Rolf hypothesized that fetal tissue might be needed to produce the other, more elusive pancreatic cell types.

Amanda Andersson-Rolf specializes in cultivating organoids from both mouse and human cells to gain a deeper understanding of the organs they are designed to replicate.
Katarína Balážová
“In development, this is where all these [cell] lineages are formed,” she explained. “The pancreas is a quiescent organ, where in adulthood you don’t have the constant self-renewal like the intestine. So, the stem cells [present] during development are the ones that you more or less live with.” Andersson-Rolf’s experiments, published in Cell, identified a stem cell that differentiates into the three cell types, enabling the researchers to create an organoid that mimicked the fetal pancreas.3 This organoid model offers new insights into pancreatic development and could ultimately advance regenerative therapies and drugs for pancreatic diseases.
To create her organoid model, Andersson-Rolf obtained first- and second-trimester samples through a collaborator's connection with an abortion clinic. However, when she cultured the cells, the standard media used for human adult pancreatic organoids proved ineffective. She quickly realized that she needed to concoct media that was just right.
“That’s where luck comes in,” she said. Unsure which developmental timepoints could generate embryonic stem cells, she cultivated 18 human fetal pancreatic organoid (hfPO) lines from various gestational weeks (GWs) and tested roughly 40 growth factor cocktails to determine the most effective conditions for growth. Among these, she found that in only one specific medium, cultures from 14–16 GWs formed budding organoids, a promising sign of their potential for development. Notably, Andersson-Rolf also tried to grow adult organoids in this fetal organoid media, but it didn’t have the same effect.
To determine whether these fetal organoids were distinct from adult organoids, the team used mRNA sequencing (mRNA-seq) and differential expression analysis for acinar, ductal, and endocrine markers. They found that organoids from 15–16 GWs samples cultured in standard adult media only expressed ductal cell markers, while organoids grown in the fetal media expressed all three markers.
This finding excited Andersson-Rolf, but it also presented another challenge. Acinar cells are notoriously hard to culture long-term, and some mature acinar cells can transdifferentiate into ductal cells. To determine whether the organoid followed the same developmental patterns as the actual pancreas, the researchers tagged two acinar cell genes with fluorescent proteins at early expansion and followed the differentiation of these cells. Not only did the cells differentiate into acinar cells, but mRNA-seq data also revealed an uptick in genes related to the functions of this particular cell type, including an increase in digestive enzyme transcripts. The team then applied the same approach to endocrine cell development, observing higher expression of endocrine markers and mRNA-seq results showing an increase in pancreatic hormones.
These organoids exhibited promising expression of the three main cell types, but Andersson-Rolf wanted to determine whether they derived from a single type of progenitor cell or multiple. The researchers hypothesized that a progenitor cell capable of differentiating into acinar, ductal, and endocrine cells—referred to as tripotent—was responsible. To investigate, they used single-cell RNA sequencing to map the cells in fetal tissue and organoids into distinct clusters. While the three cell types formed distinct clusters, the researchers saw a fourth cluster containing a previously unknown progenitor cell type. “At this point, [we thought] these cells could be special.”
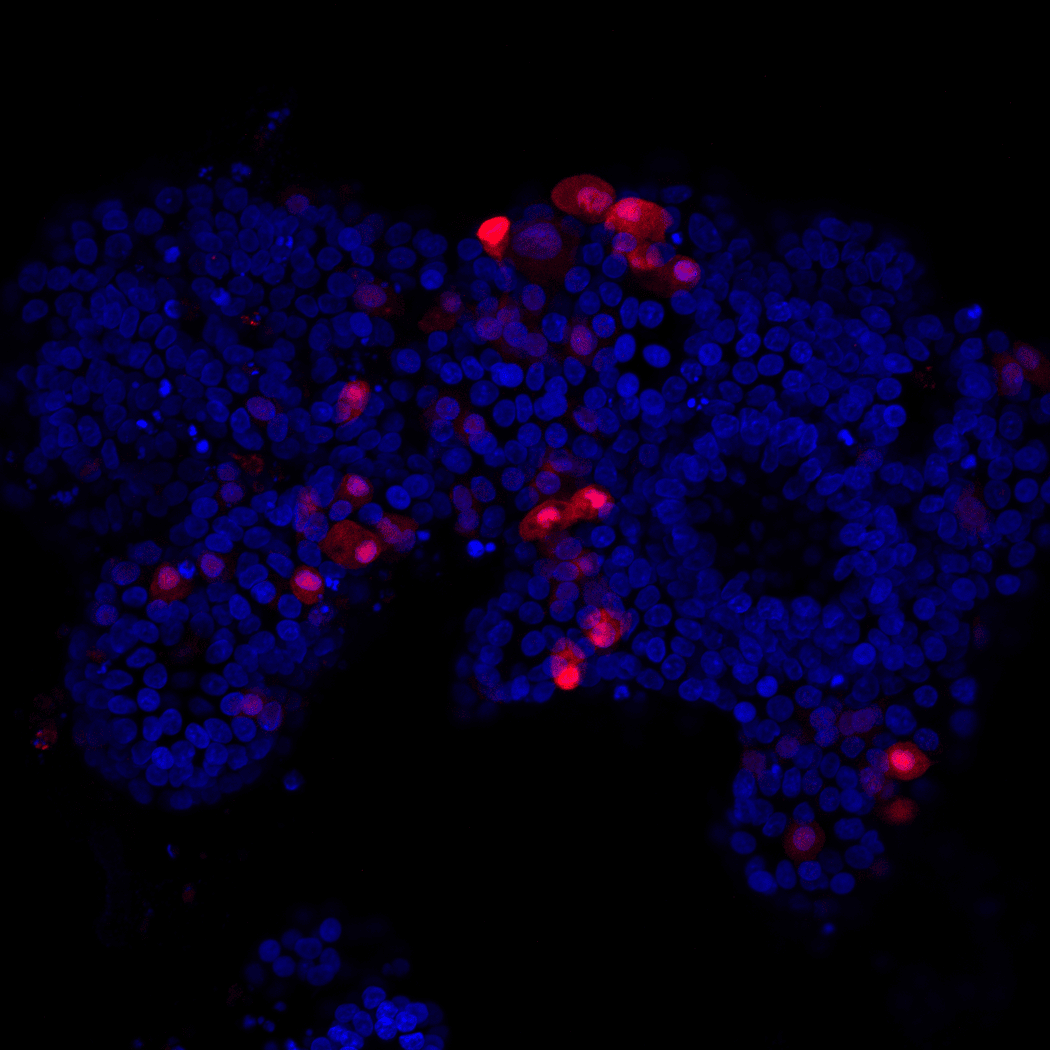
Andersson-Rolf initially set out to create fetal pancreatic organoids containing endocrine cells. She evaluated cell differentiation using different stains, marking the nuclei in blue and the endocrine cells in red.
Amanda Andersson-Rolf and Kelvin Groot
Next, Andersson-Rolf set out to characterize the progenitor cell type by analyzing stem cell markers to determine which ones conferred tripotency. When the team subclustered the cells, they found leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), a protein that marks stem cells in various tissues like the colon, was present. This intrigued Andersson-Rolf, who noted that LGR5 is present in human pancreatic stem cells but absent in mice, underscoring a key difference between human and mouse pancreatic development. This tripotent progenitor present in fetal tissue was the key to creating this new organoid model to mimic the human fetal pancreas.
“This paper shows how human development is different from a mouse,” said Pei Wang, a cellular biologist at the University of Texas Health Science Center at San Antonio, who was not involved in this study. She noted that while fetal tissue can be difficult to obtain, this work is a good resource. “It can serve as a gold standard reference to other cell lines such as induced pluripotent stem cells.”
“A majority of the pancreas field has focused on endocrine beta cells that produce insulin because of diabetes,” said Andersson-Rolf. However, with this new model, she is excited to have a platform to explore understudied cell types, such as acinar cells. These cells can develop into carcinomas, including pancreatic ductal adenocarcinoma—a highly aggressive form of pancreatic cancer. The model could help researchers understand how these cells interact during development and disease, providing better insights into cancer progression and novel therapeutic strategies.
- Huch M, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32(20):2708-2721.
- Boj SF, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1):324-338.
- Andersson-Rolf A, et al. Long-term in vitro expansion of a human fetal pancreas stem cell that generates all three pancreatic cell lineages. Cell. 2024;187(26):7394-7413.e22.















