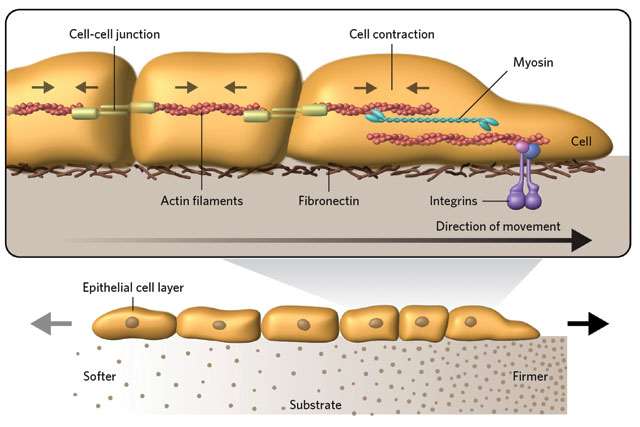
 PULLING THROUGH: Groups of human epithelial cells migrate toward firmer ground due to linkages between cells and traction with the substrate. Cells adhere to their neighbors through intercellular junctions, which are connected to myosin motor proteins within each cell. The myosin motors cause the cells to contract and tug on one another, but cells residing atop firmer substrate get a better grip through integrin proteins and are therefore able to pull the group in their direction. © GEORGE RETSECK. ADAPTED FROM SUNYER ET AL., SCIENCE, 353:1157, 2016. REPRINTED WITH PERMISSION FROM AAAS
PULLING THROUGH: Groups of human epithelial cells migrate toward firmer ground due to linkages between cells and traction with the substrate. Cells adhere to their neighbors through intercellular junctions, which are connected to myosin motor proteins within each cell. The myosin motors cause the cells to contract and tug on one another, but cells residing atop firmer substrate get a better grip through integrin proteins and are therefore able to pull the group in their direction. © GEORGE RETSECK. ADAPTED FROM SUNYER ET AL., SCIENCE, 353:1157, 2016. REPRINTED WITH PERMISSION FROM AAAS
 The paper
The paper
R. Sunyer et al., “Collective cell durotaxis emerges from long-range intercellular force transmission,” Science, 353:1157-61, 2016.
The physical features of a cell’s surroundings—the texture of a substrate and the push-pull of intercellular forces—have often taken a back seat to scientific interest in the chemical environment of cells. But biologists are finding time and again that physical signals matter. In 2000, researchers observed that fibroblasts embedded in a gel migrate toward areas where the gel is stiffest—an apparent response to physical rather than chemical signals termed durotaxis. The discovery turned heads. The report attracted almost 2,000 citations over the next decade and a half, and it became a cornerstone of the emerging field of mechanobiology, says Xavier Trepat, who studies durotaxis at the Institute for Bioengineering of Catalonia in Spain.
Exactly how cells “durotax” has been a mystery. Available research implicates durotaxis in a number of important cellular processes, from neuron development to cancerous tumor formation, but “there are only a few papers out there proposing mechanisms,” Trepat says, none of which give a definitive explanation.
Trepat and his colleagues studied the problem using traction force microscopy, which involves embedding fluorescent nanoparticles into a gel substrate and tracking the particles’ displacement. As cells drag themselves around on the gel substrate, they leave a record of the forces they exert, allowing researchers to decode the physics at play.
When Trepat and ...













