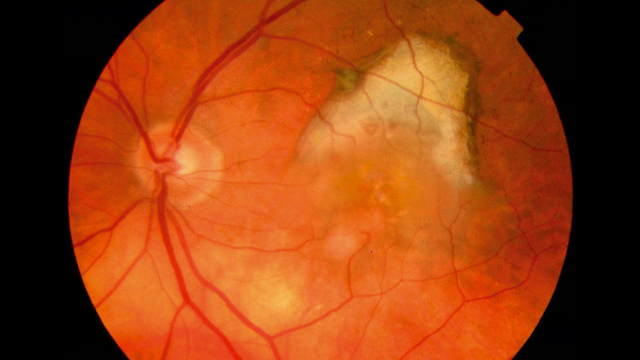
 Wet age-related macular degenerationFLICKR, COMMUNITY EYE HEALTHWet age-related macular degeneration (AMD) occurs when blood vessels grow and leak fluid into the macula, the central portion of the retina. If left untreated, this condition can lead to vision loss. A new gene therapy that blocks vascular endothelial growth factor (VEGF), a protein that plays a central role in the development of the disease, may be a safe treatment, according to a study published yesterday (May 16) in the Lancet.
Wet age-related macular degenerationFLICKR, COMMUNITY EYE HEALTHWet age-related macular degeneration (AMD) occurs when blood vessels grow and leak fluid into the macula, the central portion of the retina. If left untreated, this condition can lead to vision loss. A new gene therapy that blocks vascular endothelial growth factor (VEGF), a protein that plays a central role in the development of the disease, may be a safe treatment, according to a study published yesterday (May 16) in the Lancet.
Researchers at Johns Hopkins and their collaborators conducted an open-label Phase I clinical trial of a new treatment, in which a viral vector expressing the gene for sFLT01, a protein that neutralizes VEGF, is injected into patients’ eyes. The team enrolled 19 patients over age 50 with advanced wet AMD and divided subjects into five groups that received different doses of the gene.
“Even at the highest dose, the treatment was quite safe. We found there were almost no adverse reactions in our patients,” study coauthor Peter Campochiaro, a professor of ophthalmology at Johns Hopkins University, said in a statement. The injections reduced fluid levels and improved vision in six participants.
In five patients where the treatment did not reduce fluid levels, researchers discovered pre-existing ...


















