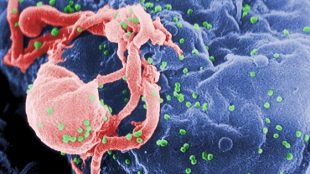
 Scanning electron micrograph of HIV-1 budding (in green) from cultured lymphocyte WIKIMEDIA COMMONS, CDC-C. GOLDSMITH
Scanning electron micrograph of HIV-1 budding (in green) from cultured lymphocyte WIKIMEDIA COMMONS, CDC-C. GOLDSMITH
Earlier this month, an advisory panel for the US Food and Drug Administration (FDA) recommended the approval of the widely used antiretroviral drug Truvada for the prevention of HIV transmission. Although clinical trials of Truvada have demonstrated a 90 percent efficacy in preventing the sexual transmission of HIV when taken daily, ScienceNOW reported, the panel’s decision to approve the drug for use in uninfected people was met with considerable resistance by experts who worry that incorrect use of the drug will lead to risky behavior as well as widespread drug resistance.
"I think it will be a catastrophe for HIV prevention in this country," Michael Weinstein, president of the AIDS Healthcare Foundation and the most vocal opponent of the recent decision, told ABC News. "Men ...












