Spatial biology is reshaping how researchers study cancer by revealing the architecture and complexity of tumors in extraordinary detail. Through techniques that combine protein- and gene-level mapping, scientists can analyze cellular behavior and decipher the intricacies of tumors and their surrounding microenvironments. The integration of AI and multiomics accelerates this progress, pushing the field toward more personalized and effective therapeutic strategies.
In this Innovation Spotlight, Traci Degeer, the director of Advanced Staining Innovation at Leica Biosystems, discusses the power of a spatial multiomics approach when studying the tumor microenvironment.
Why is spatial biology important in cancer research?
Spatial biology is becoming transformative due to its ability to show complex architecture and cellular interactions within tumors. This adds to our understanding of the tumor microenvironment, helping researchers understand how a tumor might respond to a particular treatment. Specifically, combining RNA and protein expression data helps provide a more complete picture.
How do scientists typically generate spatial biology data in the tumor microenvironment?
There are a number of ways scientists can generate spatial data in the tumor microenvironment. Depending on the amount of data needed, this data can come from protein expression or probes. Once the tissue is labeled, it is imaged, the data is acquired, and a variety of computational analysis can be performed.
Do these analysis techniques have any constraints?
There are still several critical processes that rely heavily on human expertise. For instance, formulating hypotheses and designing experiments demand a high level of creativity and domain-specific knowledge that machines currently cannot replicate. Similarly, while AI and machine learning can handle large-scale data analysis, interpreting the results of experiments often requires human insight to draw meaningful conclusions and understand broader implications. When experiments do not proceed as expected, troubleshooting typically necessitates human intervention to diagnose issues and implement effective solutions.
How does a multiomics approach enhance scientists' understanding of cancer?
Multiomics provides a comprehensive and holistic view of tumor biology by integrating multiple layers of cellular information. Each omics layer contributes unique insights into different aspects of cellular function. Genomics uncovers mutations and structural variations in DNA, offering a foundational understanding of genetic alterations. Transcriptomics reveals gene expression patterns, shedding light on how genes are regulated and expressed in cancer cells. Proteomics identifies the abundance and modifications of proteins, which are the functional molecules driving cellular processes. Finally, metabolomics captures the metabolic changes occurring within cancer cells, reflecting shifts in biochemical activity. Together, these layers create a multidimensional picture of tumor behavior and progression.
A good example of multiomics in cancer research is the MOSAIC Project, which is building a large spatial multiomics dataset in oncology by integrating six data modalities, including spatial transcriptomics, single-cell RNA sequencing, bulk RNA sequencing, whole exome sequencing, digitized histology, and clinical data. Ultimately, this is laddering up to support the development of more personalized therapies, and by using AI to enhance the analysis, they will help identify cancer subtypes, biomarkers, drug targets, and more.
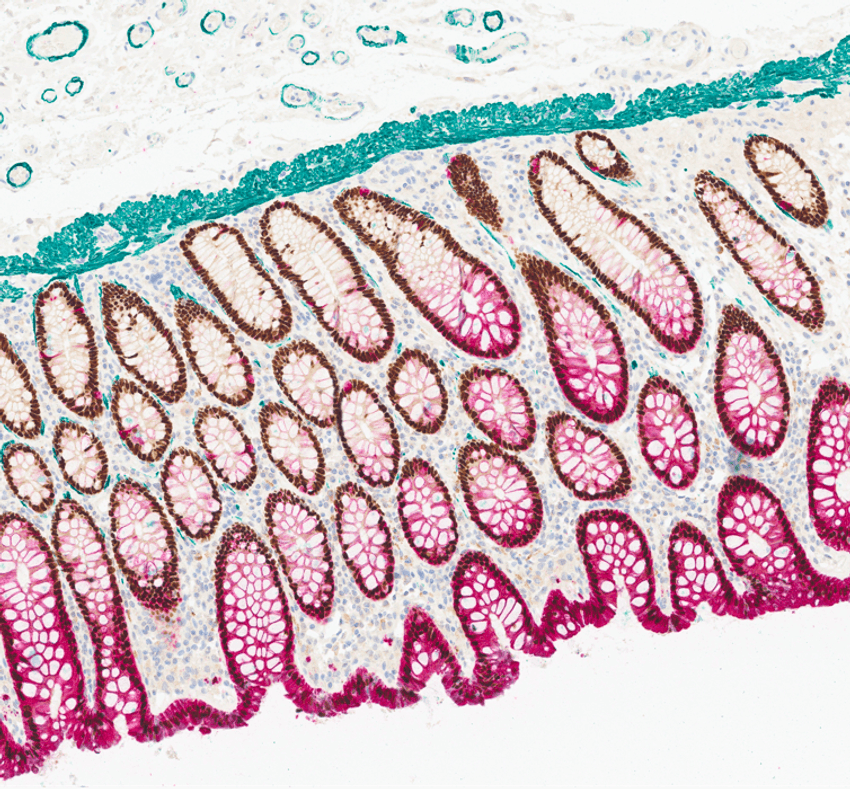
Spatial biology platforms that support the simultaneous detection of different proteins within a tissue section provide a detailed view of protein expression and localization.
Leica Biosystems
Which emerging technologies will take spatial analyses to the next level?
Several advanced technologies such as AI-driven spatial analytics are driving progress in spatial analysis, offering deeper insights into tissue organization and cellular interactions. Another example, high-resolution spatial transcriptomics, enables visualization at subcellular resolution. This allows researchers to observe the spatial distribution of transcripts within the tissue architecture. Additionally, platforms that support the simultaneous detection of dozens of proteins in tissue sections provide a detailed view of protein expression and localization.
In the clinical space, how can spatial biology techniques enhance diagnostics and disease management?
Spatial biology techniques are paving the way for enhanced diagnostics and disease management by enabling high-resolution mapping of molecular and cellular interactions within tissues, leading to more precise disease classification, targeted therapies, and real-time monitoring of treatment response.
In your opinion, what is the next frontier for spatial biology?
AI-integration of spatial data with other multiomic data will help improve treatment planning, monitoring, and patient outcomes.