 YANQI YE
YANQI YE
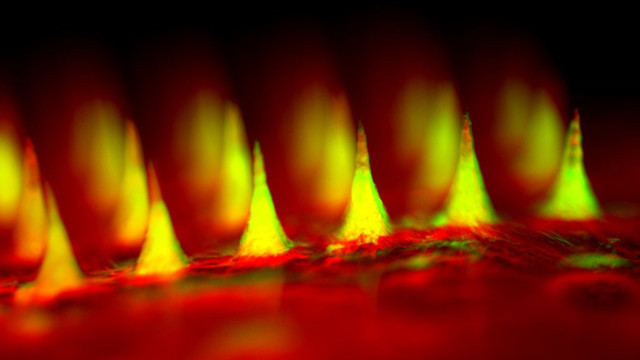
The device: A patch containing 121 microneedles loaded with specialized nanoparticles designed to release insulin when glucose levels are high could make insulin delivery a little bit “smarter,” according to a study published today (June 22) in PNAS.
“Our microneedles are smart microneedles. They can respond to glucose levels and respond at the right time,” said study coauthor Zhen Gu, a biochemical engineer at the University of North Carolina at Chapel Hill (UNC). “We are trying to mimic the function of the pancreatic beta cells,” which tightly control insulin levels in the body by keeping tabs on blood glucose concentrations.
Gu and his UNC and North Carolina State University colleagues demonstrated that the insulin-containing particles, which are exposed to blood capillaries when the microneedles puncture ...


















