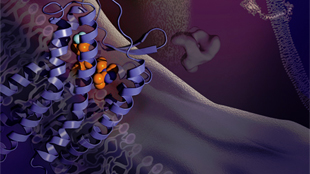
 CCR5 bound to MaravirocWU LAB, SIMMAfter a six-year struggle, a team of Chinese scientists has produced a three-dimensional portrait of CCR5, a human protein that allows HIV to invade host cells.
CCR5 bound to MaravirocWU LAB, SIMMAfter a six-year struggle, a team of Chinese scientists has produced a three-dimensional portrait of CCR5, a human protein that allows HIV to invade host cells.
This detailed snapshot, in which CCR5 is attached to the HIV drug Maraviroc, provides important clues about how HIV infections begin and how they might be stopped. “Our hope is to lay a foundation for the next generation of anti-HIV drugs,” said Beili Wu from the Chinese Academy of Sciences in Shanghai, who led the study. Her team’s results were published today (September 12) in Science.
“It’s very exciting,” said James Hoxie from the University of Pennsylvania, who studies how HIV interacts with human proteins and was not involved in the study. “For the first time, we can look at structures that are the keys to how the virus homes in on particular cell types. That’s what infection is all about.”
HIV infections begin when ...














