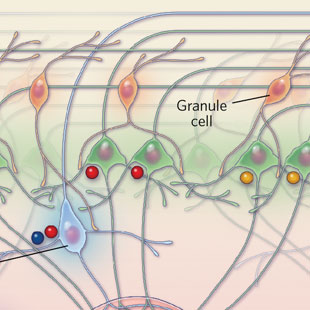
 PARSING SMELL: Within every glomerular module, olfactory sensory neurons (OSN) activated by a particular range of odorants connect to a network of deeper, downstream neurons. Juxtaglomerular cells, located in the first layer (1), respond to a wider range of odorants than tufted cells, neurons in the next layer that relay signals to higher brain areas (2). Mitral cells, a type of neuron located in the deepest layer of the module, respond to even fewer odorants (3). The particular set of odorants that activate the mitral cells depends on the lateral location of each cell within this layer.
PARSING SMELL: Within every glomerular module, olfactory sensory neurons (OSN) activated by a particular range of odorants connect to a network of deeper, downstream neurons. Juxtaglomerular cells, located in the first layer (1), respond to a wider range of odorants than tufted cells, neurons in the next layer that relay signals to higher brain areas (2). Mitral cells, a type of neuron located in the deepest layer of the module, respond to even fewer odorants (3). The particular set of odorants that activate the mitral cells depends on the lateral location of each cell within this layer.
View full size JPG | PDF© SCOTT LEIGHTON
The paper
S. Kikuta et al., “Odorant response properties of individual neurons in an olfactory glomerular module,” Neuron, 77:1122-35, 2013.
Although the human sense of smell is feeble compared to that of many animals, it is acute enough to distinguish between very similar odors. Researchers know a lot about how our 400 or so distinct types of odor receptors combine to differentiate roughly 10,000 odors. But the neuronal architecture underlying our ability to precisely discriminate between slightly different odorant molecules picked up by the same receptor is less well understood.
In humans, the outer layer of the olfactory bulb, the most forward part of the brain, which lies atop the back of the nasal passage, is comprised of roughly 5,500 ball-like neural junctions called glomeruli. ...





















