Lymphatic filariasis, a devastating parasitic disease transmitted by mosquitoes, affects more than 110 million people worldwide.1 People who suffer from the infection experience extremely painful edema with thickening of skin and underlying tissue, which can lead to permanent disability. There is no rapid cure for the infection, and most of the current treatments do not effectively target adult worms. Furthermore, these treatments are not suitable for children and pregnant women.
To develop new treatments for the microscopic roundworms that cause this debilitating disease, chemist Andrés Palencia knew he needed to get creative. Palencia, whose work at the Institute for Advanced Biosciences focuses on the structural biology of novel therapeutic targets, explored a relatively underutilized approach. Instead of targeting the parasitic worms themselves, Palencia turned his attention to the worm microbiome. “There is a lot of research going on in human microbiota,” said Palencia. “But the microbiota is [also] really important for some pathogens.”
Back in the 1970s, researchers first discovered that filariasis-causing parasitic worms housed Gram-negative bacteria within their cells; further studies showed that most filarial worms that infect people depend on this bacterium, called Wolbachia, for their growth and survival.2 Leveraging this, Palencia’s team developed a therapy to inhibit Wolbachia's growth disrupting its symbiosis with its worm host.3 The results, published in Science Advances, highlight the potential of targeting key elements of the microbiome in disease-causing organisms as a promising approach to controlling parasitic infections in humans.
Researchers have previously demonstrated that broad-spectrum antibiotics can deplete Wolbachia bacteria, subsequently reducing the burden of filarial worms in mouse models.4, However, these drugs can also disrupt the human microbiome by killing beneficial bacteria, so researchers are searching for therapeutics that selectively inhibit Wolbachia growth.
However, it is difficult to identify new and specific targets in Wolbachia, said Palencia, mainly because it is challenging to grow the bacterium in the lab. “It’s symbiosis with the host is so strong, that you will have to grow the host along with Wolbachia,” Palencia explained. This makes it more difficult to use genetic tools to manipulate the bacterial genome.
To circumvent this challenge, Palencia’s team turned to a previously-described in vitro model that uses Wolbachia-infected insect cells for high-throughput screening of drug molecules. Using this system, the researchers could observe Wolbachia within insect cells through fluorescence and easily track the effects of various drugs. They tested a library of more than 200 boron-based compounds that have previously shown antimicrobial properties and identified a handful of these compounds that were active against Wolbachia.
The team then performed further experiments to characterize the activity and efficacy of these candidates, and determined that two compounds, Cmpd6 and Cmpd9, most effectively reduced Wolbachia numbers in the cells.
Since the development of a safe and effective drug requires understanding its mechanism of action, the researchers sought to decipher how these compounds work. Palencia’s team, along with other research groups, had previously shown that boron-based compounds target bacterial Leucyl-tRNA synthetase (LeuRS), an enzyme involved in protein synthesis.5 To test whether the candidate compounds were also binding to LeuRS, the researchers purified a part of the Wolbachia protein that contained the potential drug-binding site.
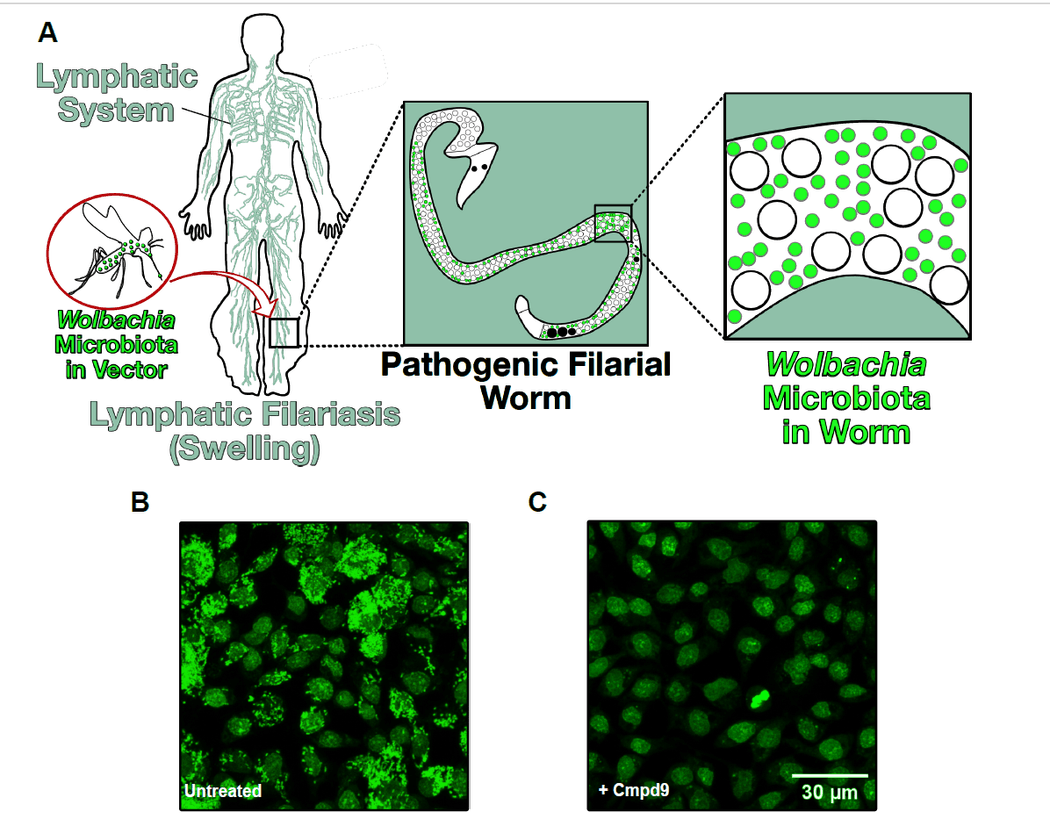
A) Filarial worms, transmitted by mosquito bites, reach lymph nodes, resulting in infection. B) Mosquito cells infected with Wolbachia (green fluorescent). C) Reduction in Wolbachia load in host cells treated with a boron-based compound.
Andrés Palencia, Guillaume Hoffmann
The researchers analyzed the binding of candidate compounds to the enzyme and found that the boron-based compounds bind to Wolbachia LeuRS with high affinity. This prevented the enzyme from binding with its natural substrate, blocking protein synthesis. X-ray crystallography revealed that the binding site is unique to Wolbachia, suggesting that these compounds may be able to target this particular bacterium, without harming other bacteria in the human microbiome
“Some specificities in the drug-binding pocket make Wolbachia unique,” said Palencia. This site is different in the bacterium and humans, which opens the potential for this drug-target combination to be specific to Wolbachia, he explained.
“This is a good piece of work that adds to the field,” said Eric Caragata, a mosquito microbiologist at the University of Florida who was not associated with the study. Although this field of microbiome research is relatively new, he thinks that such microbiome-targeting strategies will become more common in the future. “The more we know about the interactions between these [worms] and the microbiome, the better placed we are to exploit them,” he added.
However, Caragata said that further studies must test the safety and efficacy of these candidate compounds in mammalian cells and eventually live animals.
While Palencia agreed, he also pointed out that previous studies have offered some clues about this. “We know that in general, boron-based molecules that went into clinical trials have low toxicity.”
For example, the Food and Drug Administration has already approved a compound that inhibits fungal LeuRS for treatment of onychomycosis, an infection of the nail and nail bed, and another compound that inhibits the enzyme in Mycobacterium tuberculosis has recently completed Phase 2 clinical trials.6-8 Palencia’s team hopes that they can procced towards initiating clinical trials with their compounds in the next few years.
- Taylor MJ, et al. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376(9747):1175-1185.
- McLaren DJ, et al. Micro-organisms in filarial larvae (Nematoda). Trans R Soc Trop Med Hyg. 1975;69(5-6):509-514.
- Hoffmann G, et al. Targeting a microbiota Wolbachian aminoacyl-tRNA synthetase to block its pathogenic host. Sci Adv. 2024;10(28):eado1453.
- Sharma R, et al. Minocycline as a re-purposed anti-Wolbachia macrofilaricide: Superiority compared with doxycycline regimens in a murine infection model of human lymphatic filariasis. Sci Rep. 2016;6(1);23458.
- Palencia A, et al. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012;19(7):677-684.
- Sharma N, Sharma D. An upcoming drug for onychomycosis: Tavaborole. J Pharmacol Pharmacother. 2015;6(4):236.
- Hoffmann G, et al. Adenosine-dependent activation mechanism of prodrugs targeting an aminoacyl-tRNA synthetase. J Am Chem Soc. 2023;145(2):800-810.
- Diacon AH, et al. A first-in-class leucyl-tRNA synthetase inhibitor, ganfeborole, for rifampicin-susceptible tuberculosis: A phase 2a open-label, randomized trial. Nat Med. 2024;30(3):896-904.















