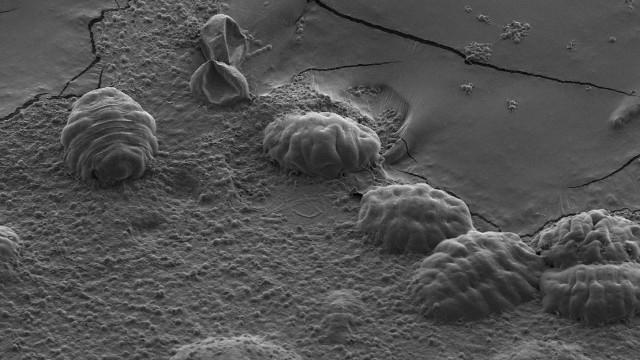
 Scanning electron micrograph of six tardigradesTHOMAS BOOTHBYHardy, microscopic animals called tardigrades, also known as water bears, can survive desiccation. Until now, it wasn’t clear exactly how. The results of a study published in Molecular Cell today (March 16) suggest that proteins lacking stable 3-D structures, called tardigrade-specific intrinsically disordered proteins (TDPs), form glass-like solids that protect the animals during drying.
Scanning electron micrograph of six tardigradesTHOMAS BOOTHBYHardy, microscopic animals called tardigrades, also known as water bears, can survive desiccation. Until now, it wasn’t clear exactly how. The results of a study published in Molecular Cell today (March 16) suggest that proteins lacking stable 3-D structures, called tardigrade-specific intrinsically disordered proteins (TDPs), form glass-like solids that protect the animals during drying.
Other organisms achieve desiccation tolerance with a sugar called trehalose, which forms glass-like solids upon drying. For years, researchers assumed that tardigrades used trehalose, too, but many species of water bears only express small amounts of the sugar—likely not enough to confer the substance’s preservative capabilities.
TDPs “seem to work by a mechanism which is similar to this sugar, trehalose,” said coauthor Thomas Boothby, a postdoc at the University of North Carolina, Chapel Hill. “It seems like evolution has basically come up with two different ways to do the same trick.”
Boothby and colleagues identified a group of TDPs during a screen for tardigrade genes upregulated during desiccation. They confirmed that many of the genes’ protein products were disordered, and that these genes were expressed ...


















