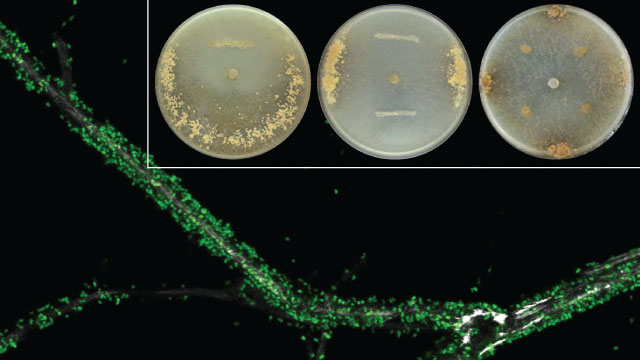
 FUNGAL FARM?: Bacteria labeled with green fluorescent protein disperse across the hyphal surfaces of the fungus Morchella crassipes. The inset shows the clumped distribution of Pseudomonas putida bacteria among the hyphal threads.SASKIA BINDSCHEDLER, UFZ LEIPZIG (CLSM IMAGE) & MARTIN PION, UNINE (Petri dishes inserts)
FUNGAL FARM?: Bacteria labeled with green fluorescent protein disperse across the hyphal surfaces of the fungus Morchella crassipes. The inset shows the clumped distribution of Pseudomonas putida bacteria among the hyphal threads.SASKIA BINDSCHEDLER, UFZ LEIPZIG (CLSM IMAGE) & MARTIN PION, UNINE (Petri dishes inserts)
Every spring, several students from the microbiology lab at the Université de Neuchâtel in Switzerland take to the woods that surround the town. Their mission is not scientific, but culinary. As part of a local mycology club, they hunt for morels, fungal delicacies prized in kitchens around the world for their earthy, nutty flavor.
Microbiology group leader Pilar Junier says that during morel season in Switzerland, restaurants everywhere advertise food prepared with morels, and “it’s delicious.” But beyond her taste for the fungus, Junier has a scientific interest in morels. She is a trained bacteriologist, but she works side by side with mycologist Daniel Job to explore the seldom-studied interactions between fungi and bacteria.
Junier and Job wanted to understand the interactions between bacteria and ...





















