Biofilms are multicellular networks that can grow almost anywhere. These slime-encased microbial colonies can survive harsh conditions and develop resistance to antimicrobial agents such as antibiotics, posing a serious risk to human health. In this summary, learn about biofilm formation, how drug-resistant biofilms contribute to the global health burden, and strategies to combat biofilms in medical settings.

Bacterial biofilms can pose a serious threat to human health, causing infections in natural tissues and on medical devices.
iStock, quantic69
What Is a Biofilm?
Almost all bacteria are capable of forming multicellular communities known as biofilms.1 Biofilms are primarily made up of live, dormant, and dead bacterial cells and held together by an extracellular matrix (ECM), usually secreted by the bacteria themselves.2 The biofilm ECM comprises a variety of biopolymers, such as DNA, proteins, lipids, and polysaccharides.3
The biofilm structure acts as a protective shield for its residents against external stressors, and also lowers the metabolic rates of the bacterial cells.5 This allows biofilm inhabitants to survive harsh conditions, including high temperatures, salinity, and pH; UV radiation; low nutrients; and antibiotic treatment.6
Biofilms are incredibly diverse, and while they are predominantly associated with bacteria, they can also include other organisms such as algae, fungi, or protozoans.6 Biofilms can grow adhered to almost any surface, including human tissues, medical devices, industrial settings, and natural environments. They can be benign, beneficial, or pathogenic.4 The latter category can cause persistent, drug-resistant infections and can secrete toxins and other harmful compounds.4
Biofilm Formation
There are several key stages of biofilm formation.
1. Attachment
Attachment is the first step in biofilm formation, during which individual bacterial cells migrate and adhere to a substrate and each other.3 This adherence is reversible; scientists also refer to this stage as reversible attachment, anchoring, or latching.
2. Colonization
If conditions are appropriate after attachment, the bacteria will begin secreting a biopolymer coating that will form the ECM.3 During this step, the adherence between the cells becomes irreversible and they form an immature biofilm or microcolony.6
Quorum sensing is an intercellular signaling mechanism that significantly contributes to biofilm formation and maturation, and virulence gene activation.7 Quorum sensing enables bacterial cells in the community to communicate with one another, co-regulating their gene expression and promoting phenotypes that facilitate the biofilm's survival and growth.8
3. Maturation
During biofilm maturation, the bacteria in the microcolony multiply and different cell populations emerge. Mature biofilms can be made up of many layers and may contain more than one microbial species.7
4. Dispersion
The mature biofilm surface layer contains bacterial cells that can escape via disruption of the microcolony wall and these cells can disperse to form new biofilm structures.9 Researchers who study biofilms often liken this to the metastatic spread of tumors; the biofilm dispersal process is sometimes called metastatic seeding.9
Antibiotic Resistance in Biofilms
Bacteria that grow in biofilms may have increased mutation rates compared to their free-floating counterparts, and they participate in higher rates of horizontal gene transfer, both of which contribute to antibiotic resistance development.10 Bacteria within biofilms can also secrete antibiotic-degrading enzymes and use membrane channels called efflux pumps to reduce the concentration of antibiotics and other harmful substances inside the bacterial cells.11
The three-dimensional biofilm structure also physically protects its residents against antibiotics and the host immune system, particularly those in the deeper layers.7 Scientists have reported biofilm inhabitants that are up to 1,000 times more tolerant to antibiotics than free-living bacteria.9
Biofilm Examples
Biofilms can grow on heart valves, in the lungs of people with cystic fibrosis, in the intestines, and on teeth, as well as on medical devices and implants, including artificial joints, stents, and catheters.7 Drug-resistant biofilms are a serious threat to human health; up to 80 percent of microbial infections in humans are biofilm-associated infections, causing significant morbidity and mortality.5
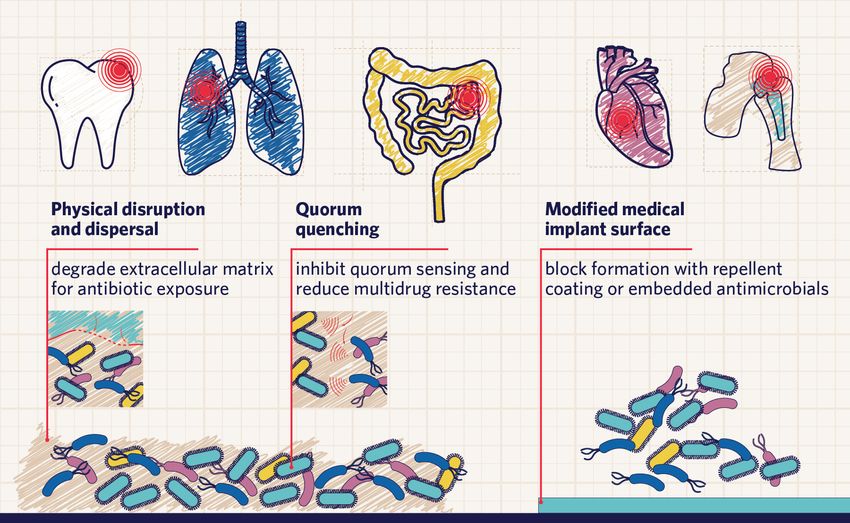
Biofilms can grow on teeth, lungs, intestines, heart valves, and medical implants. Scientists are exploring several different strategies to combat biofilms, including physical disruption of the ECM, quorum quenching, and blocking their formation in medical devices.
Modified from © istock.com, Olha Pohrebniak, mustafahacalaki; designed by Alisha Vroom
In the mouth, biofilms form dental plaque. The composition of these biofilms can have a significant impact on tooth health; beneficial bacteria present in a dental biofilm will deter colonization by detrimental species that can cause disease and cavities.2
The gastrointestinal (GI) system is also host to a delicate balance of biofilm communities. Biofilms comprising many different bacterial species naturally grow throughout the gut, with a range of effects on host health.13 Abnormal biofilm growth is associated with GI disorders, including inflammatory bowel disease, infections, and cancers.13
Biofilms are also responsible for infections in prosthetic joints, in which they can cause devastating complications. Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa all commonly form biofilm infections in artificial joints, as well as in stents, catheters, shunts, and pacemakers.8
It is worth noting that biofilms can also be enormously beneficial in non-medical settings. For example, Alcanivorax borkumensis, a bacterial species found in the ocean, can consume hydrocarbons, making them ideal for cleaning up oil spills at sea.7
Biofilm Disruptors
Researchers have found combating bacterial biofilms in medical settings to be a major challenge, largely owing to the resistance of these infections to antibiotics. One of the proposed treatment strategies is to induce biofilm dispersal via ECM degradation, making the exposed dormant bacteria vulnerable to antibiotics.3
Another strategy is to prevent biofilm formation by inhibiting the attachment process.3 This can be achieved using quorum quenching agents, which inhibit quorum sensing and lower the likelihood of multidrug resistance in the biofilm.3 Scientists are also investigating the possibility of modifying the surface of medical implants such as artificial joints, for example by adding a bacterial-repellent coating or embedding antimicrobial therapeutics.3
Scientists continue to deepen our knowledge of bacterial biofilms, how they form, and their impact on human health. This will allow researchers to develop more innovative treatment strategies to combat biofilm-related infections, improving health outcomes for a range of patients.
FAQ
What is a biofilm and what does it do?
- Biofilms are multicellular microbial networks primarily made up of live, dormant, and dead bacterial cells held together by a slimy extracellular matrix (ECM), usually secreted by the bacteria themselves. The biofilm structure protects its residents against external stressors, such as harsh environments, low nutrients, and antibiotics.
What are examples of biofilms?
- Dental plaque is one common example of a biofilm. Bacteria can also form biofilms on heart valves, in the lungs of people with cystic fibrosis, in the intestines, and on medical devices and implants, including artificial joints, stents, and catheters.
How are biofilms treated or eliminated from the body?
- Researchers are investigating methods to disperse biofilms via extracellular matrix degradation, exposing the biofilm-forming bacteria to antibiotics. Other strategies include preventing or disrupting biofilm formation with quorum quenching agents and modifying medical device surfaces with bacterial-repellent coatings or antimicrobial therapeutics.
- Zhao A, et al. Understanding bacterial biofilms: From definition to treatment strategies. Front Cell Infect Microbiol. 2023;13.
- López D, et al. Biofilms.Cold Spring Harb Perspect Biol. 2010;2(7):a000398.
- Su Y, et al. Biofilms: Formation, research models, potential targets, and methods for prevention and treatment. Adv Sci. 2022;9(29):2203291.
- Percival SL, et al. Introduction to Biofilms. In: Percival S, Knottenbelt D, Cochrane C, eds. Biofilms and Veterinary Medicine. Springer; 2011:41-68.
- Wang S, et al. Strategy to combat biofilms: A focus on biofilm dispersal enzymes. Npj Biofilms Microbiomes. 2023;9(1):1-14.
- Mirghani R, et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022;8(3):239-277.
- Shree P, et al. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med Microecol. 2023;16:100084.
- Visperas A, et al. Current treatments for biofilm-associated periprosthetic joint infection and new potential strategies. J Orthop Res. 2022;40(7):1477-1491.
- Rumbaugh KP, Sauer K. Biofilm dispersion. Nat Rev Microbiol. 2020;18(10):571-586.
- Michaelis C, Grohmann E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics. 2023;12(2):328.
- Gaurav A, et al. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors.Microbiology. 2023;169(5):001333.
- Motta JP, et al. Gastrointestinal biofilms in health and disease. Nat Rev Gastroenterol Hepatol. 2021;18(5):314-334.