A human female is born with all the egg cells she will ever have. The possibility for the development of new oocytes is zero. Given this constraint, it is crucial that these gametes remain healthy and viable for decades until they are needed to form an embryo. Irrespective of the ‘age’ of the fertilized oocyte, the resulting embryo has the characteristics of a freshly born cell, indicating the existence of mechanisms that counteract accrued cellular damage and keep the egg fresh. What are these processes that drive the prolonged life of human egg cells?
Elvan Böke, an oocyte biologist at the Centre for Genomic Regulation, studies exactly that. A healthy cell boasts vigilant scanning for and removal of misfolded, damaged, or unnecessary proteins. A common feature associated with cellular aging is the breakdown of intracellular protein degradation machinery.1 In previous studies done in mouse oocytes, Böke and other researchers found that these cells rely on two key adaptations to keep their cytoplasm free of harmful clutter: They store and degrade of protein aggregates in vesicles and contain oocyte proteins with exceptionally long lives.2,3
However, how human oocytes maintain protein homeostasis remains unclear. Extrapolating from the mice data, scientists believed that human oocytes rev up protein degradation to remain healthy over time. In a new study, Böke and her team overturned this theory and showed that human oocytes in fact dial down their degradative activities and dampen metabolism to minimize cellular stress over their protracted lifespan.4 The researchers published these findings in The EMBO Journal. The improved understanding of mechanisms underlying human oocyte longevity could lead to better reproductive therapeutics.
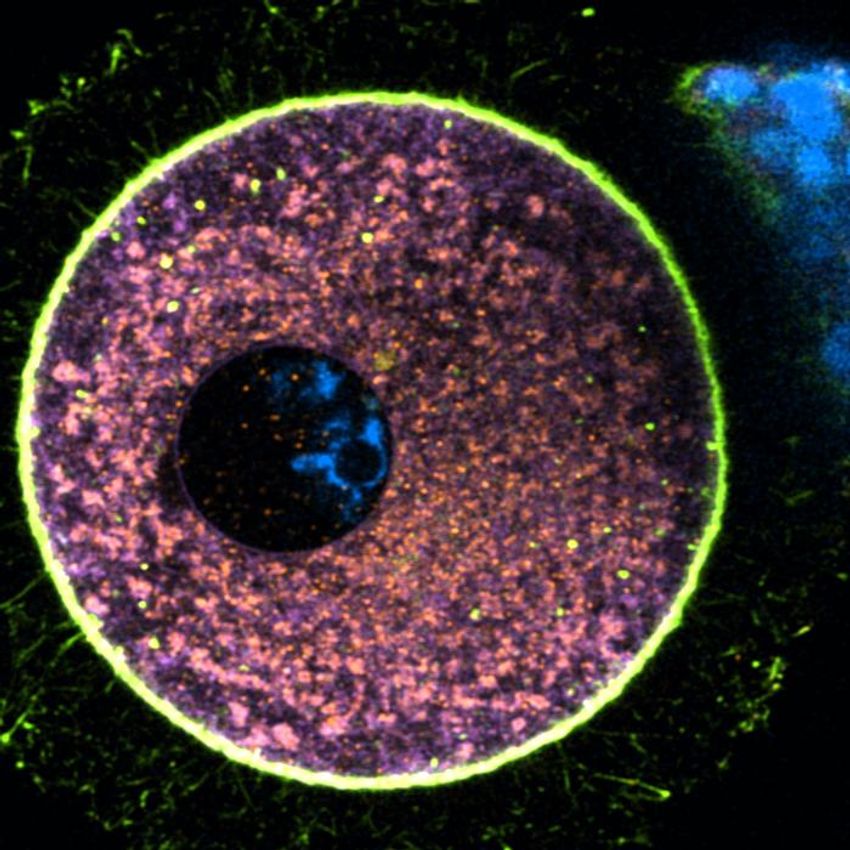
Elvan Böke, an oocyte biologist at the Centre for Genomic Regulation, and her team collected human oocytes to study how protein degradation contributes to the prolonged life of these cells. Shown here is an immature human oocyte labelled with intracellular markers of mitochondria (orange), endoplasmic reticulum (purple), and actin (green).
Gabriele Zaffagnini, Centre for Genomic Regulation
“By looking at more than a hundred freshly donated eggs, the largest dataset of its kind, we found a surprisingly minimalist strategy that helps the cells stay pristine for many years,” Böke said in a statement.
The researchers first looked at how human oocytes degrade proteins, focusing on two major waste disposal pathways—proteasomal and lysosomal. They collected over 100 healthy human oocytes from 21 donors; samples included both immature eggs that are incapable of being fertilized and fertilization-ready mature ones. To visualize the proteolytic vesicles, the team injected the oocytes with dyes that labeled lysosomes and proteasomes. For comparison, the team also analyzed somatic cells attached to the oocytes. Contrary to the rodent data, they observed a decrease in both proteasomal and lysosomal activities of human oocytes during maturation. Böke and her team hypothesized that this could either be a result of naturally lower oocyte protein levels or their reduced degradation. To tease apart their findings, the researchers labeled proteasomal and lysosomal components with antibodies in immature and mature oocytes. They saw that mature eggs had lower abundance of lysosomes and proteasomes as compared to the immature ones, which indicated that these cells power down their degradation machinery. The team also observed a surprising increase in the accumulation of the lysosomal dye in the cell membranes, pointing to elevated extracellular transport of these vesicles.
Since the researchers manipulated donated human eggs, they wanted to confirm that the experimental conditions did not affect their health and skew these results. To do this, they performed a dye-based assessment of the gametes’ mitochondrial membrane potential—a measure of the metabolic state—and observed no changes in cells’ potentials. However, the team noticed a dip in the values as the eggs matured, suggesting that the cells become less active as they get ready to be fertilized.
What happens to the proteins ear-marked for disposal in the face of this cellular adaptation? To answer this question, Böke and her team labeled protein aggregates using a dye—they observed that they get packaged up into lysosomes. They speculated that these could be the membrane-adjacent vesicles that are transported out of the oocytes for further processing.
Böke posits that the low organelle activity of human oocytes points to their low metabolic rate, thus preventing the accumulation of harmful components and reactive oxygen species. These findings could lead to better fertility-improving techniques and provide a deeper understanding of what goes wrong when these methods fail. “Fertility patients are routinely advised to take random supplements to improve egg metabolism, but evidence for any benefit for pregnant outcomes is patchy,” Böke said. “By looking at freshly donated eggs we’ve found evidence to suggest the opposite approach, maintaining the egg’s naturally quiet metabolism, could be a better idea for preserving quality.”
- Pohl C, Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366(6467):818-822.
- Zaffagnini G, et al. Mouse oocytes sequester aggregated proteins in degradative super-organelles. Cell. 2024;187(5):1109-1126.e21.
- Harasimov K, et al. The maintenance of oocytes in the mammalian ovary involves extreme protein longevity. Nat Cell Biol. 2024;26(7):1124-1138.
- Zaffagnini G, et al. The proteostatic landscape of healthy human oocytes. EMBO J. 2025:1-20.