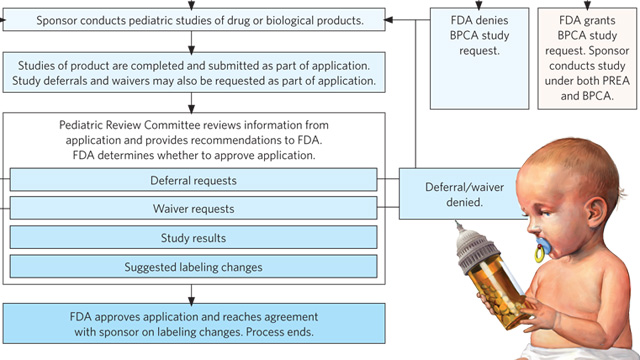
 Infographic: How the Laws Work
Infographic: How the Laws Work
View full size JPG | PDFMATT COLLINS (BABY); US GOVERNMENT ACCOUNTABILITY OFFICE REPORT, "PEDIATRIC RESEARCH," MAY 2011
The Pediatric Research Equity Act (PREA) of 2003 requires that companies developing new drugs that could be used to treat a condition in children perform clinical trials in kids before winning FDA approval. The Best Pharmaceuticals for Children Act (BPCA) of 2002 offers financial incentives for companies to test drugs, either pre- or post-approval, in pediatric trials deemed necessary by the FDA.















