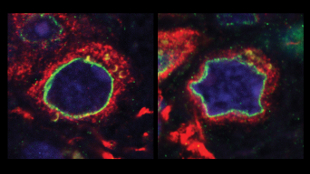
 Altered nuclear morphology within brain tissue sample of Parkinson’s patient with a mutation in LRRK2 (right), as compared to cells of a healthy, age-matched control (left). Image courtesy of Merce Marti and Juan Carlos Izpisua Belmonte"There’s a new suspect in the search for the causes of Parkinson’s disease—deformities in the nuclear membrane of neural stem cells. Scientists observed the same defects, caused by a single gene mutation, in brain tissue samples from deceased Parkinson’s patients, suggesting that nuclear deterioration—and the mutation that drives it—could play a role in the pathology of the disease. The study, published today (October 17) in Nature, also shows that correcting the mutation reverses this phenotype, pointing to new ways to treat this cause of neurodegeneration.
Altered nuclear morphology within brain tissue sample of Parkinson’s patient with a mutation in LRRK2 (right), as compared to cells of a healthy, age-matched control (left). Image courtesy of Merce Marti and Juan Carlos Izpisua Belmonte"There’s a new suspect in the search for the causes of Parkinson’s disease—deformities in the nuclear membrane of neural stem cells. Scientists observed the same defects, caused by a single gene mutation, in brain tissue samples from deceased Parkinson’s patients, suggesting that nuclear deterioration—and the mutation that drives it—could play a role in the pathology of the disease. The study, published today (October 17) in Nature, also shows that correcting the mutation reverses this phenotype, pointing to new ways to treat this cause of neurodegeneration.
“I don’t recall anyone ever suggesting this as a major phenotype [for Parkinson’s], so that’s really quite a big new direction for the field,” said Mark Cookson, a neuroscientist at the National Institutes of Health in Bethesda, Maryland, who did not participate in the study.
Parkinson’s disease has traditionally been attributed to a loss of dopamine-generating neurons, which leads to the degenerative muscle control that is characteristic of the disease. But Parkinson’s also causes many other sensory problems, which cannot be explained by a dopaminergic mechanism.
Over the past 5 years, several groups have shown that disruption of the structure of the nuclear envelope—the lipid bilayer that separates nucleus from cytoplasm—is correlated with aging and certain age-related pathologies in the human brain, though the precise ...













