Ovarian cancer is one of the deadliest gynecological cancers due to most of the cases—approximately 80 percent—being diagnosed in the late stages of the disease.1 The standard of care for ovarian cancer, which is platinum-based chemotherapy and surgery, has not advanced in more than 30 years. But that may be about to change.
Researchers at the immunotherapy company IMUNON recently reported results in Gynecologic Oncology from their Phase 1/2 clinical trial testing their DNA-based immunotherapy in women with advanced-stage ovarian cancer.2 They found that their drug, which delivers the gene for the cytokine interleukin-12 (IL-12), directly to the ovarian tumors, led to a 13 month increase in overall survival of patients than those on the standard of care. They are currently recruiting patients to test the drug in a pivotal Phase 3 trial.

Stacy Lindborg is the CEO of the immunotherapy biotechnology company IMUNON.
IMUNON
“We were overwhelmed with the magnitude of the effect,” said Stacy Lindborg, the chief executive officer at IMUNON. “We observed an overall survival benefit that has never been seen in the frontline ovarian cancer space.”
IL-12 Kickstarts the Immune System to Turn Cold Ovarian Tumors Hot
Ovarian tumors sustain themselves by creating a “cold” or immunosuppressive environment around themselves. This makes it difficult for the immune system or immunotherapies to target the cancer for killing. The researchers, led by Khursheed Anwer, who is now the executive vice president and chief science officer of IMUNON, wanted to find a way to make ovarian tumors recognizable to the immune system, so they turned to the decades of research on IL-12.
“IL-12 is a potent signaling molecule, and it connects…the two major arms of the immune system: adaptive and innate immunity,” said Lindborg.
IL-12 enhances the proliferation and activation of T cells and natural killer cells against tumors, and it also increases the production of interferon gamma, which is another cytokine that enhances immune responses in the tumor microenvironment. IL-12 also inhibits tumor angiogenesis, which is the process by which tumors grow new blood vessels to support themselves.
“It's really very well predicated [and] understood in the literature and, therefore, becomes a really phenomenal mechanism of action to be bringing forward in clinical development,” said Lindborg.
The catch, however, is that historically, when researchers tried to use IL-12 as an immunotherapy, they delivered it systemically in the blood. This led to liver and blood toxicities that made it unsafe to use as a therapy.3
But the team at IMUNON developed a way to direct IL-12 specifically to ovarian tumors. They the researchers encapsulated a DNA plasmid encoding the gene for IL-12 inside a synthetic nanoparticle engineered to get into the nucleus. They called the plasmid-nanoparticle drug IMNN-001. The researchers delivered IMNN-001 into the peritoneal cavity, where the ovaries are, through a catheter. When they looked at the cells in the tumor microenvironment, the researchers saw that both healthy and cancerous cells took up the therapy and began producing IL-12.
“[It’s] having the immune system actually bring to bear what would be naturally occurring in our body, but we're allowing it to be really released on…this cancer that these women are fighting,” said Lindborg.
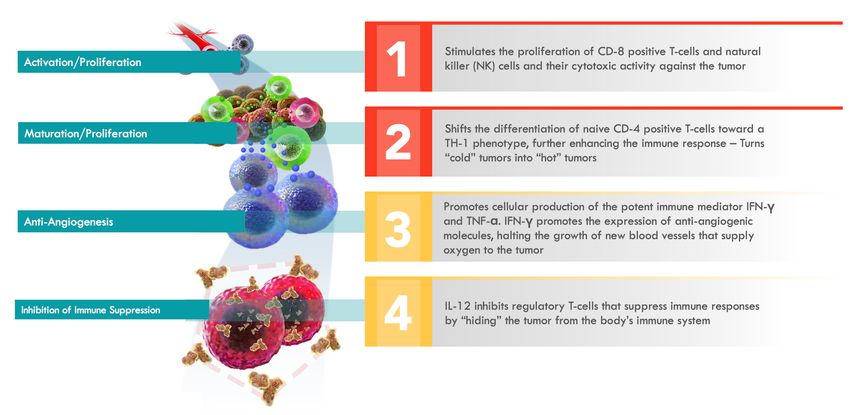
IMUNON’s IL-12-based therapy, called IMNN-001, stimulates the immune system against ovarian cancer cells.
IMUNON
From Phase 3 Trials to a Potentially New Standard of Care
In May 2025, researchers from IMUNON presented the results of their Phase 1/2 trial toward the end of the American Society of Clinical Oncology (ASCO) meeting in a surprisingly full conference room.
They presented their findings that IMNN-001 led to a median overall survival of 13 months more than the standard of care in patients with Stage 3 and 4 ovarian cancer. A subset of patients with homologous recombination deficiency (HRD) positive tumors, meaning that they had mutations in genes like BRCA1 or 2, responded even better to the therapy.
After the presentation, they quickly received positive feedback. “It was a physician that was reflecting on how enormous of a milestone this was for ovarian cancer, and that is so enriching to hear,” said Lindborg. “It's been certainly very rewarding, and I think it just gives us more of an external validation, objective validation, of our own belief that this truly could be transformative to women fighting ovarian cancer.”
She added that after the presentation multiple attendees asked about the upcoming Phase 3 trial testing IMNN-001 in advanced ovarian cancer patients and whether their patients could participate.
For the Phase 3 trial, the IMUNON team will follow the same protocol and trial design as the Phase 1/2 study, but Lindborg said that they have added a biomarker to stratify the patients. Because of how well patients with HRD positive tumors did on the therapy, the team will have each tumor screened to determine whether it has mutations that make it HRD positive or not. They are currently recruiting patients to the trial.
“It's so fulfilling to know that we have a very genuine chance at transforming the standard of care,” said Lindborg. “It's such an honor to be in this place and to have this opportunity before us.”
- Siegel RL, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
- Thaker PH, et al. OVATION-2: A randomized phase I/II study evaluating the safety and efficacy of IMNN-001 (IL-12 gene therapy) with neo/adjuvant chemotherapy in patients newly-diagnosed with advanced epithelial ovarian cancer. Gynecologic Oncology. 2025;197:182-191.
- Jia Z, et al. IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Front Immunol. 2022;13:952231.













