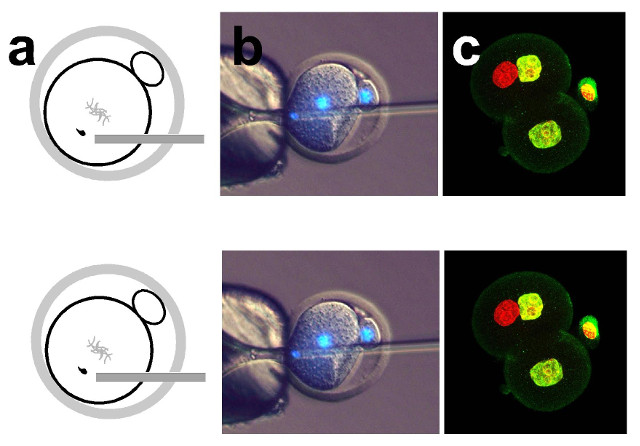
 Diagram showing sperm injection into a one-cell embryo in the phICSI method (a); microscopy (with DNA stained blue) of sperm being injected into a one-cell mouse embryo, which has a diameter of about 0.1 mm (b); some of the resulting two-cell embryo cells have a sperm-derived genome (red) and an egg-derived one (yellow), which can develop to form offspring (c)UNIVERSITY OF BATHWhen a sperm fertilizes an egg, it transforms from a fully differentiated cell into half the genetic material of an embryo. This transformation is called reprogramming. For centuries, scientists thought that only eggs could effect this change in sperm, University of Bath biologist Anthony Perry told The Scientist. For the first time, Perry and colleagues have created viable mouse embryos starting not with eggs but with mitotic cells, they reported in a study published today (September 13) in Nature Communications. Many of these embryos developed to term; the resulting pups were healthy and, later, able to breed viable offspring themselves.
Diagram showing sperm injection into a one-cell embryo in the phICSI method (a); microscopy (with DNA stained blue) of sperm being injected into a one-cell mouse embryo, which has a diameter of about 0.1 mm (b); some of the resulting two-cell embryo cells have a sperm-derived genome (red) and an egg-derived one (yellow), which can develop to form offspring (c)UNIVERSITY OF BATHWhen a sperm fertilizes an egg, it transforms from a fully differentiated cell into half the genetic material of an embryo. This transformation is called reprogramming. For centuries, scientists thought that only eggs could effect this change in sperm, University of Bath biologist Anthony Perry told The Scientist. For the first time, Perry and colleagues have created viable mouse embryos starting not with eggs but with mitotic cells, they reported in a study published today (September 13) in Nature Communications. Many of these embryos developed to term; the resulting pups were healthy and, later, able to breed viable offspring themselves.
The team “shows that eggs that have already begun embryonic development, and are about to divide from the one-cell to two-cell stage, still retain the ability to ‘remodel’ sperm DNA,” Hugh Clarke, a developmental biologist at McGill University in Montreal who was not involved in the work, told The Scientist in an email. “So this ability is not restricted to eggs at the time of fertilization.”
“This is the first time that anyone has been able to demonstrate that embryos can reprogram a differentiated cell of any kind,” said Perry. The discovery raises the possibility that other mitotic cell types might also be able to reprogram sperm. If so, Perry added, “it might one day mean that we could generate embryos from other cell types—perhaps ...



















