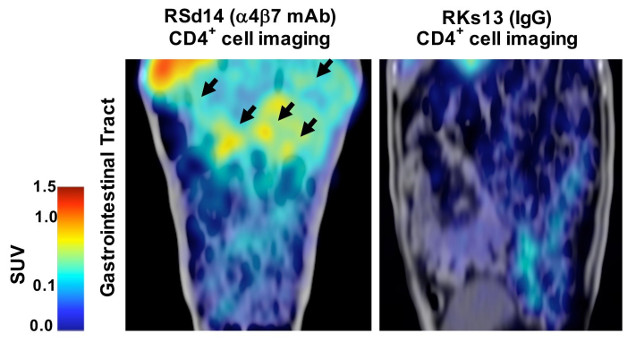
 Immuno-PET/CT analysis confirms the preservation of CD4+ cells during a4b7 antibody treatment. SCIENCE, S. BYRAREDDY ET AL. Treatment with an antibody that blocks T cell migration to the gut eliminates detectable viremia in simian immunodeficiency virus (SIV)-infected Rhesus macaques withdrawn from antiretroviral therapy (ART), according to a study published today (October 13) in Science. The effect continued for 18 weeks after the last intravenous antibody infusion.
Immuno-PET/CT analysis confirms the preservation of CD4+ cells during a4b7 antibody treatment. SCIENCE, S. BYRAREDDY ET AL. Treatment with an antibody that blocks T cell migration to the gut eliminates detectable viremia in simian immunodeficiency virus (SIV)-infected Rhesus macaques withdrawn from antiretroviral therapy (ART), according to a study published today (October 13) in Science. The effect continued for 18 weeks after the last intravenous antibody infusion.
“With standard retroviral drugs, once you withdraw them, the virus comes back. And here is a case where the virus isn’t coming back,” said viral immunologist David Montefiori of Duke University in North Carolina who was not involved in the work.
The antibody binds a molecule on T cells called α4β7, which directs T cells to gut lymphoid tissues “like a zip code on the cells,” explained study coauthor Aftab Ansari, a pathologist at Emory University in Atlanta. Because the gut is a major site of viral replication and CD4+ T-cell infection in SIV and HIV, Ansari said, “if these cells are trafficking into the gut, they are basically adding fuel to fire.”
Ansari and colleagues at the National Institute of Allergy and Infectious Diseases ...




















