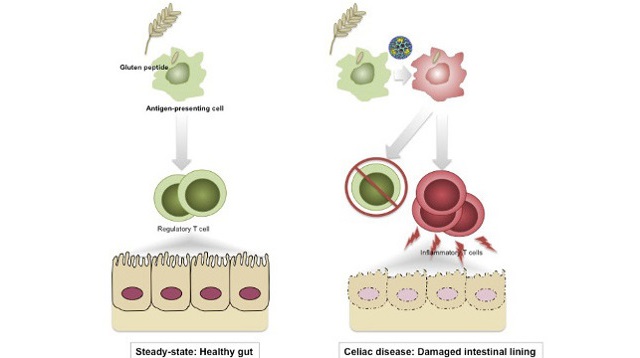
 Left: Normally, a regulatory immune response blocks inflammation to dietary antigens. Right: When reovirus penetrates at the site where dietary gluten is present, it induces danger signals that confuse the immune system and lead it to develop an aggressive inflammatory immune response against gluten, which may trigger celiac disease.UNIVERSITY OF CHICAGO; VALÉRIE ABADIE, BANA JABRIOrally infecting mice with a human reovirus resulted in an immune response against gluten and led to symptoms of celiac disease in the rodents, researchers reported today (April 6) in Science. Reoviruses are prevalent in humans; while children are commonly infected with them, reoviruses are not known to cause disease in people. But the results of this mouse study suggest that a reovirus infection may spur development of celiac disease in certain individuals.
Left: Normally, a regulatory immune response blocks inflammation to dietary antigens. Right: When reovirus penetrates at the site where dietary gluten is present, it induces danger signals that confuse the immune system and lead it to develop an aggressive inflammatory immune response against gluten, which may trigger celiac disease.UNIVERSITY OF CHICAGO; VALÉRIE ABADIE, BANA JABRIOrally infecting mice with a human reovirus resulted in an immune response against gluten and led to symptoms of celiac disease in the rodents, researchers reported today (April 6) in Science. Reoviruses are prevalent in humans; while children are commonly infected with them, reoviruses are not known to cause disease in people. But the results of this mouse study suggest that a reovirus infection may spur development of celiac disease in certain individuals.
“It’s been hypothesized for decades that virus infection can trigger autoimmune processes. This study provides an example of that phenomenon and some mechanistic insight into how this might work for celiac disease,” said Herbert Virgin, a virologist at the University of Washington, who has collaborated with some of the study’s authors but was not involved in the present work.
An inflammatory immune response develops in the guts of individuals with celiac disease when they ingest foods containing gluten, a protein found in wheat. People with celiac disease have a genetic predisposition to gluten intolerance, but prior epidemiological data provided hints that environmental factors, including viral infections, are also associated with initiation of the disorder.
To test the role of a human reovirus in stimulating celiac ...



















