Within the leaves and stems of unlucky plants, pathogens from the Xanthomonas genus wage microscopic war with a secret weapon: transcription activator like effectors (TALEs). The microbial invaders secrete these proteins to activate genes in plant cells to promote microbial survival.1
First identified in 1989 as individual proteins involved in pathogen virulence, TALEs and the response that some plants developed in response to them captured the interest of plant geneticists.2 By the mid-2000s, TALE researchers knew that the proteins possessed a nuclear localization signal, a transcriptional activation domain, and a domain with multiple, mostly repetitive blocks of 33-35 amino acids, today called the repeat-variable diresidues (RVDs), that promoted gene expression.3-6
However, how these regions corresponded to specific sequences of DNA remained elusive. Plant scientists suspected that understanding this mechanism could help identify targets during disease progression, improving counter measures to protect crops. When researchers unveiled how TALEs selected their DNA targets, it catapulted these molecular machines into the wider research audience as tools for gene editing, eventually setting the stage for a revolution in the field.
The Origin of TALENs: Cracking the Code
In 2007, Jens Boch, at the time a research scientist in Ulla Bonas’s group at the Martin-Luther-University Halle-Wittenberg, was troubleshooting a problem with his colleague: Two different TALE proteins activated the same gene. “That’s when I said, basically, ‘why not look at it as if one of those RVDs recognized one base’,” he recalled.

Julien Valton leads a research team at Cellectis using TALEN technology to improve gene therapies, including cell therapies like CAR T cells.
Cellectis
The duo wrote out the repeat sequences one under the other and noticed a pattern: While most of the 34 amino-acid sequence was the same, the 12th and 13th residue differed between the repeats. Sensing that they may have discovered the solution to the TALE sequence specificity, the group experimentally tested their findings. Over and over again, they could reliably predict which sequences a given TALE would bind just by looking at those two amino acids in the RVDs.
“I couldn’t sleep for two nights,” said Boch, today a plant geneticist at the Leibniz University Hannover, as he recalled his excitement. “It was immediately clear that you can reprogram the protein to go to any specific location in a genome.”
Around the same time, plant geneticist Adam Bogdanove, then at Iowa State University, and his graduate student Matthew Moscou reached the same conclusion using computational methods. Having visited Boch’s group earlier in the year, Bogdanove wrote to them of their findings. The two groups published their respective results in Science in October of 2009. The TALE code was cracked.7,8 “That was an exciting time,” Boch said.
TAL effectors revolutionized the field of DNA targeting, CRISPR democratized it.
—Adam Bogdanove, Cornell University
Shortly after this publication, Bogdanove struck up a collaboration with Daniel Voytas, a plant geneticist at the University of Minnesota. Voytas was interested in developing genome editing tools for plants. In 2009, the leading technology for this was zinc finger domains, but they were difficult to construct.9
Voytas and Bogdanove fused FokI, the endonuclease used in zinc finger domains, to TALEs to create TALE nucleases (TALENs).10 “It became something that everybody was interested in,” said Bogdanove, who today works at Cornell University. “Everyone who had historically had an interest in genome editing started to look at TALEs and started to engineer them.” Subsequent groups further improved upon these novel nucleases by removing unnecessary elements from the protein and identifying domains that simplified base recognition.11,12
“The whole community of genome editing was absolutely excited, because it was so difficult to make zinc finger nucleases work specifically,” Boch explained. By contrast, with the TALE code cracked, TALENs were more straightforward.
TALENs also became more popular than zinc finger domains or another gene editing tool, meganucleases, for a less technical reason: Voytas and Bogdanove made their method and reagents available on a resource sharing site, Addgene. “This kit that Dan Voytas made and shared with the world was actually really critical to getting people to use these things,” said Charles Gersbach, a biomedical engineer at Duke University. Additionally, several websites, including one developed by Bogdanove, further helped researchers create custom TALENs for their application.13
At the time, Gersbach had recently started his group at Duke University studying zinc finger nucleases. He recalled thinking that the new TALENs sounded interesting, but he was skeptical if they would work. When one of his students asked to work on TALENs, Gersbach said that he recommended they focus on their current projects with zinc finger nucleases. However, “He went and worked on TALEs anyway and got it to work super fast, and the results were great,” Gersbach said.
Gersbach and his team began using TALENs in their research on genome editing therapies for Duchenne muscular dystrophy.14 “What was most exciting about [TALENs] is how it brought in so many people into the gene editing field,” Gersbach said.
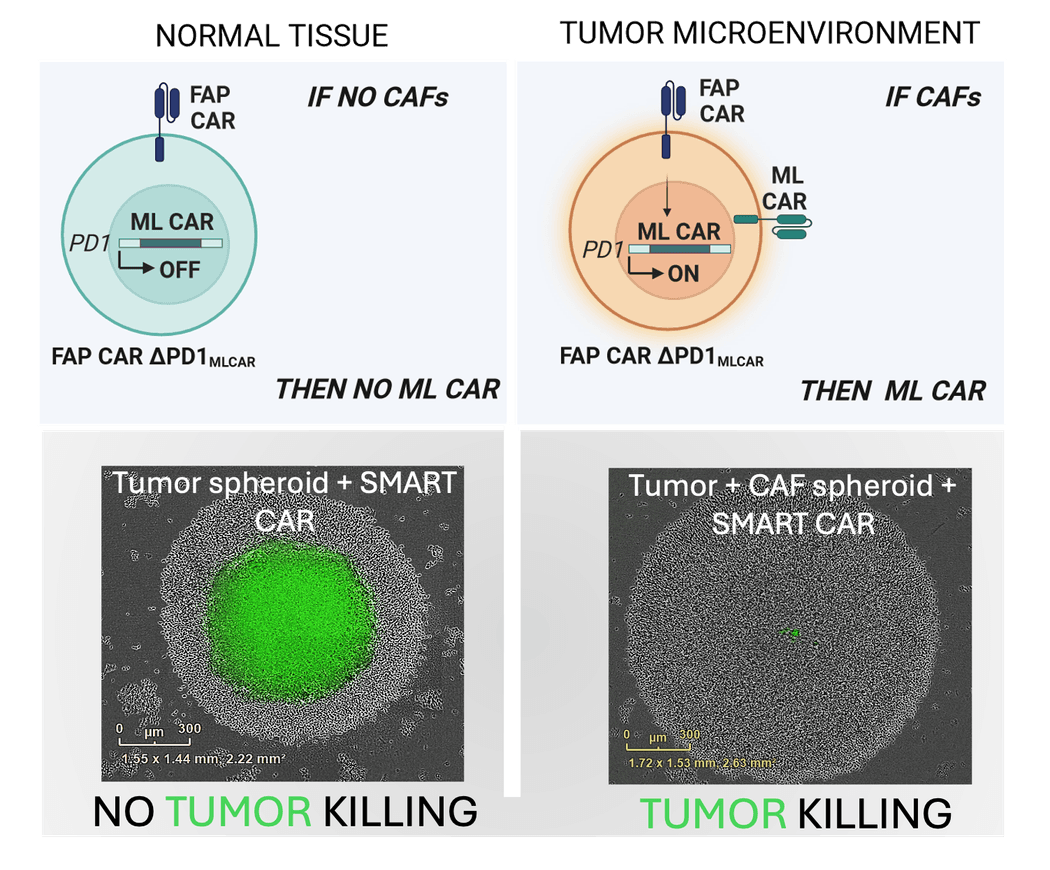
Researchers at Cellectis used TALEN gene editing to create a SMART CAR T cell that responded to its environment. A graphic abstract (top) and fluorescent image (bottom) shows this CAR T cell expressing a tumor-antigen specific CAR only when it was in the tumor (green) environment to exert tumor-killing.
Created in BioRender. Valton, J. (2024) https://BioRender.com/e77u997.
The interest in TALENs extended beyond academic research. Recombinetics, a bioengineering company focused on animal gene editing, produced the first hornless dairy cattle with the new technology.15 Then, Calyxt, a plant-based biotechnology company used TALENs to produce the first commercially available genome-edited plant.16 “It was the beginning of the genome editing revolution,” Boch said.
TALENs Expand CAR T Cell Therapy
In the early 2000s, Cellectis, a company developing genome editing technologies, used meganucleases to selectively alter genome in cells.17 Although these tools sometimes displayed low efficiency in edited the cell population, the enhanced precision and safety for molecular tools at the time excited the researchers.18 “We were popping up the champagne at the time,” said Julien Valton, a cell engineer and the vice president of the gene therapy team at Cellectis.
When TALENs entered the science scene, they expanded genome editing efficiency, with the first generation of these editors successfully editing up to 25 percent of the cells.19 Later versions accomplished efficiencies of 76 or almost 100 percent for some target edits.20,21 “Can you imagine the revolution at that time?” Valton recalled. “Before the TALEN era we were pretty happy with something that was not therapeutically or biotechnologically relevant. When the TALEN was developed, it became like a reality.”
In 2011, the University of Minnesota exclusively licensed the TALEN patent to Cellectis. One team at the company applied it to projects to improve another emerging therapy for cancer, chimeric antigen receptor (CAR) T cells. In CAR T cell therapy, a patient’s cells are isolated and genetically engineered to recognize a specific tumor antigen and then returned to the patient as a cancer-fighting drug.
Continue reading below...
This therapy proved to be successful in multiple patients, however, researchers recognized that while using an individual’s own cells avoided the risk of their immune systems’ attacking the therapeutic cells, it introduced its own limitations.22,23 Some patients do not have sufficient numbers of T cells to donate. Having a population of T cells from a healthy donor that could be used in multiple patients would greatly expand the utility of CAR T cell therapy, but this required making the T cells invisible and nonreactive to their new host.
Cellectis approached this challenge by using TALENs to remove the gene for the T cell receptor (TCR) from their donated T cells, creating universal CAR T (UCART) cells.24 In 2015, one team used TALENs to generate UCART cells that recognized an antigen on B cells to treat acute lymphoblastic leukemia in two infants in a compassionate use case.25 Subsequently, in 2020 and 2022, these UCART cells demonstrated safety and efficiency in two Phase I clinical trials.26,27
TALENs as a Launching Pad for CRISPR Genome Editing
Only a few years after TALENs entered research labs, the CRISPR Cas9 system in bacteria was demonstrated as an RNA-driven endonuclease and was quickly incorporated as a gene editing tool.28 Although a huge advancement by itself, Gersbach said that, thanks to TALENs, “Gene editing had gotten so much more attention that people knew what to do with CRISPR when it showed up.”
What was most exciting about [TALENs] is how it brought in so many people into the gene editing field.
—Charles Gersbach, Duke University
But CRISPR did have a big advantage over previous gene editors. “CRISPR is so much easier to implement. And this is the TALE guy saying this,” Bogdanove said. Each RVD for the base pair in the target sequence must be assembled to one another in a stepwise fashion, and researchers needed to construct two TALENs, whereas for CRISPR Cas9, it cut DNA as a single unit.
“The large size and the repetitive nature could make [TALENs] difficult to deliver in certain cases,” Gersbach said. But, in some contexts, TALENs offered an advantage. For instance, until recently, CRISPR systems could not efficiently localize to the mitochondria, so TALENs were predominantly used to edit mitochondrial DNA in models of disorders associated with this organelle, including using CRISPR-free base editing.29-31
“For the certain instances where people solved those problems and got it working well, the technology can be very effective, and so for those that have gotten it working well and invested in it, they're still pursuing it today,” said Gersbach.
For example, Valton said that TALENs offer the best balance of safety and precision for his team’s research. Although the proteins can be bulky, which complicates their production and delivery, Valton said that the increased targeting reduces the likelihood of the TALENs cutting off their target. Additionally, unlike CRISPR, which requires a specific sequence to be near the cut site, the Cellectis team can create a TALEN to any specific loci they wish.
Recently, the team used this feature to apply their UCART cell design to create an inducible therapeutic cell that is only active within the tumor.32 Using TALENs, they replaced a suppressive gene with the sequence for a tumor antigen CAR. “This is a very good example of creating a CAR T cell that can sense its environment, target what needs to be targeted, and spare what can be spared,” said Shipra Das, an immunooncologist at Cellectis who develops CAR T therapies using TALEN technology.
Next, the team is exploring gene therapy applications beyond CAR T cells. “We just feel the first tremor of this field, and stay tuned for the future, because it's going to be super, super, super exciting,” Valton said.
“At the end of the day, TAL effectors were a huge leap forward in engineering for DNA binding specificity,” Bogdanove said. “TAL effectors revolutionized the field of DNA targeting, CRISPR democratized it.”
- Marois E, et al. The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol Plant Microbe Interact. 2002;15(7):637-646.
- Bonas U, et al. Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria. Mol Gen Genet. 1989;218:127-136.
- Szurek B, et al. Type III-dependent translocation of the Xanthomonas AvrBs3 protein into the plant cell. Mol Microbiol. 2002;46(1):13-23.
- Zhu W, et al. AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol Plant Microbe Interact. 1998;11(8):824-832.
- Vivian A, Arnold DL. Bacterial effector genes and their role in host-pathogen interaction. J Plant Pathol. 2000;82(3):163-178.
- Kay S, et al. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;318(5850):648-651.
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326(5959):1501.
- Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509-1512.
- Ramirez CL, et al. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods. 2008;5(5):374-375.
- Christian M, et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186(2):757-761.
- Miller JC, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29(2):143-148.
- Lamb BM, et al. Directed evolution of the TALE N-terminal domain for recognition of all 5′ bases. Nucleic Acids Res. 2013;41(21):9779-9785.
- Doyle EL, et al. TAL effector-nucleotide targeter (TALE-NT) 2.0: Tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40(W1):W117-W122.
- Ousterout DG, et al. Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchenne muscular dystrophy patients. Mol Ther. 2013;21(9):1718-1726.
- Carlson DF, et al. Production of hornless dairy cattle from genome-edited cell lines. Nat Biotechnol. 2016;34(5):479-481.
- Menz J, et al. Genome edited crops touch the market: A view on the global development and regulatory environment. Front Plant Sci. 2020;11:586027.
- Chames P, et al. In vivo selection of engineered homing endonucleases using double-strand break induced homologous recombination. Nucleic Acids Res. 2005;33(20):e178.
- Grizot S, et al. Efficient targeting of a SCID gene by an engineered single-chain homing endonuclease. Nucleic Acids Res. 2009;37(16):5405-5419.
- Miller JC, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29(2):143-148.
- Cade L, et al. Highly efficient generation of heritable zebrafish gene mutations using homo- and heterodimeric TALENs. Nucleic Acids Res. 2012;40(16):8001-8010.
- Beddel VM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114-118.
- Lamers CHJ, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J Clin Oncol. 2006;24(13):e20-e22.
- Melenhorst JJ, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022;602(7897):503-509.
- Poirot L, et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75(18):3853-3864.
- Qasim W, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):eaaj2013.
- Benjamin R, et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): A phase 1, dose-escalation trial. Lance Haematol. 2022;9(11):e833-e843.
- Benjamin R, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: Results of two phase 1 studies. Lancet. 2020;396(10266):1885-1894.
- Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-821.
- Bacman SR, et al. mitoTALEN reduces the mutant mtDNA load in neurons. Mol Ther Nucleic Acids. 2024;35(1):102132.
- Hashimoto M, et al. MitoTALEN: A general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol Ther. 2015;23(10):1592-1599.
- Mok BY, et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583(7817):631-637.
- Dharani S, et al. TALEN-edited allogeneic inducible dual CAR T cells enable effective targeting of solid tumors while mitigating off-tumor toxicity. Mol Ther. 2024;32(11):3915-3931.