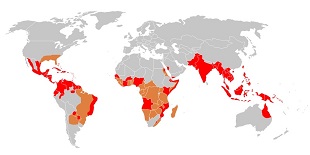
 Map showing regions populated by the virus’ vector, the Aedes aegypti mosquito (red and orange), and regions with an active epidemic of dengue fever (red only).WIKIMEDIA, PERCHERIEDengue is the most common mosquito-transmitted virus in the world and, in severe infections, can cause potentially life-threatening fevers. Currently, only one vaccine has been approved, but now a new, attenuated vaccine is performing well in randomized, double-blind trials, according to a study published today (March 16) in Science Translational Medicine.
Map showing regions populated by the virus’ vector, the Aedes aegypti mosquito (red and orange), and regions with an active epidemic of dengue fever (red only).WIKIMEDIA, PERCHERIEDengue is the most common mosquito-transmitted virus in the world and, in severe infections, can cause potentially life-threatening fevers. Currently, only one vaccine has been approved, but now a new, attenuated vaccine is performing well in randomized, double-blind trials, according to a study published today (March 16) in Science Translational Medicine.
“We developed and tested a human challenge model of dengue virus infection,” study coauthor Steven Whitehead of the National Institute of Allergy and Infectious Diseases told reporters during a press briefing. “The really remarkable success of this endeavor is now the subject of [this paper].”
There are four serotypes of the flavivirus (DEN-1 up to DEN-4) that can cause disease. “A useful vaccine really needs to protect against all four of them at the same time,” explained Whitehead in the statement. Using recombinant DNA technology, the researchers created various candidate vaccines for each dengue type that were tested individually in clinical trials to confirm their ability to stimulate an immune response. The researchers then mixed the best-performing vaccines for each serotype into a tetravalent vaccine—“a ...






















