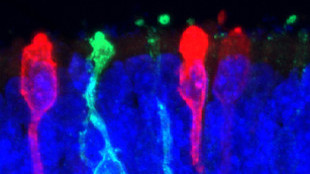
 Photoreceptors derived from human iPS cells X. ZHONG. C. GUTIERREZ AND M.V. CANTO-SOLER AT THE WILMER EYE INSTITUTE, JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINEResearchers have taken another step toward creating a functional human retina in the laboratory. Previous studies showed that an early-stage retina, including photoreceptors with primary cilia and parts of the inner segment structure, can be generated in culture from induced human pluripotent stem cells (iPSCs). Now, in a paper published today (June 10) in Nature Communications, Maria Valeria Canto-Soler, director of the retinal degeneration research center at Johns Hopkins University and her colleagues demonstrate the ability to grow the most mature retinal tissue from iPSCs yet: the in vitro product was able to develop functional photoreceptor cells.
Photoreceptors derived from human iPS cells X. ZHONG. C. GUTIERREZ AND M.V. CANTO-SOLER AT THE WILMER EYE INSTITUTE, JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINEResearchers have taken another step toward creating a functional human retina in the laboratory. Previous studies showed that an early-stage retina, including photoreceptors with primary cilia and parts of the inner segment structure, can be generated in culture from induced human pluripotent stem cells (iPSCs). Now, in a paper published today (June 10) in Nature Communications, Maria Valeria Canto-Soler, director of the retinal degeneration research center at Johns Hopkins University and her colleagues demonstrate the ability to grow the most mature retinal tissue from iPSCs yet: the in vitro product was able to develop functional photoreceptor cells.
“The major advance here is the ability to make retinal cells that can respond to light and that form into what appears to be remarkably proper orientation,” said Bruce Conklin, a senior investigator with the Gladstone Institute of Cardiovascular Disease at the University of California, San Francisco, who was not involved with the study.
The miniature human retinal tissue was able to form the outer-segment discs that are essential for light-sensing and contained all seven retinal cell types, including the four types of photoreceptor cells that express opsins, the transmembrane proteins that transfer captured photons into a physiological sensory response to light.
While others have also developed systems to study the human retina in the lab, the current study extends these capabilities, according to coauthor ...














