Deciphering protein spatial organization is essential for understanding complex disorders such as cancer. To better study cellular machinery important for health and disease, scientists can employ optoproteomics to discover proteins in their native subcellular environments.
Recently, a research team from the University of Stuttgart utilized this new technology to analyze triple-negative breast cancer (TNBC) cells and identify factors affecting the subcellular organization of Polycomb Repressive Complex 2 (PRC2).1 This regulator of transcriptional repression can be mislocalized in TNBC cells, leading to enhanced metastasis.2

Nikhil Rao, PhD
Chief Commercial Officer
Syncell
In this Innovation Spotlight, Nikhil Rao, Syncell’s chief commercial officer, examines the utility of Microscoop®, the company’s optoproteomics platform and its key role in TNBC research.
What is optoproteomics?
Spatial proteomics is a rapidly growing field that helps scientists understand proteins within the spatial context of tissues or cells. With this information, researchers discover, quantify, and learn how specific proteins influence disease, drug treatment, or cell biology function.
Various tools within this space can help researchers understand proteins and their interactions. Our Microscoop technology, termed optoproteomics, uses automated photo-biotinylation for high-precision, microscopy-guided proteomic discovery. It enables researchers to achieve unbiased discovery without knowing which proteins exist a priori.
How does the Microscoop platform work?
The Microscoop system photolabels proteins with similar morphological features at user-defined regions of interest (ROls), defined by fluorescence staining. The size of these features can reliably decrease to 350 nm in resolution. The system utilizes directed photochemistry by shining a fixed wavelength of light at each ROI, one field of view (FOV) at a time for up to tens of thousands of FOVs across the sample. This photolabeling covalently tags proteins with biotin nonspecifically within the ROI. Photolabeled proteins are then extracted and sent to a mass spectrometry instrument to reveal ROI-specific known and novel proteins associated with subcellular structures such as organelles that are critical to biological processes and disease.
This technology allows users to answer questions that couldn’t be answered before, by profiling receptors, junctions, membranes, organelle compartments, or even entire cells that are responsible for disease, treatment, pharmacological agent trafficking, and developmental biology.
How does the Microscoop technology compare to other spatial biology techniques?
Existing spatial proteomics tools rely on the use of antibodies to profile predefined proteins, thus not allowing for true novel protein constituent discoveries. Syncell’s Microscoop technology is the only tool that enables high-precision, unbiased, spatial proteomic discovery in subcellular tissues or cells. The technology helps researchers achieve unbiased discovery in spatial proteomics at disease sites, with the ability to accurately discover new protein components from the exact location where specific biological processes occur.
In what fields is Microscoop having an impact?
The technology has proven relevance across a broad spectrum of applications including, but not limited to, biomarker discovery in amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, and TNBC. Additionally, we have been able to uncover the proteins at the T-B cell synapse interface that are responsible for immune regulation. Further, one can understand trafficking of material through vesicles, condensates, transmembrane proteins, and much more. With this information, researchers can identify site-specific novel proteins to develop new drugs and better diagnostics.
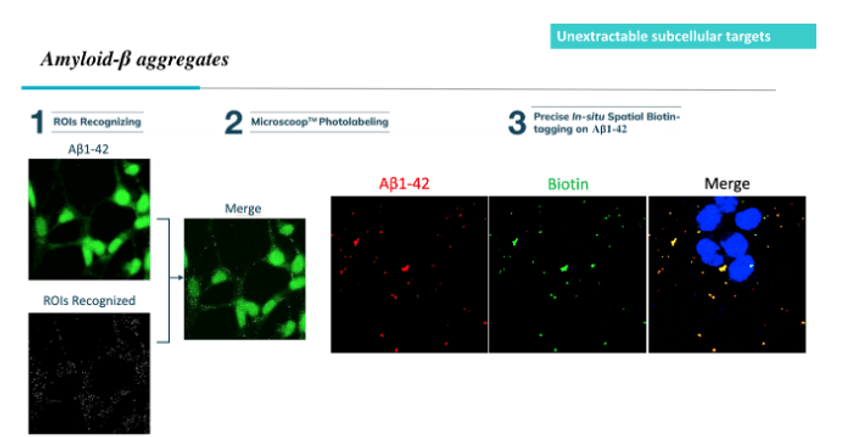
Using optoproteomics to photolabel amyloid-beta peptides in a region of interest (ROI) allows researchers to precisely biotinylate target peptides in situ and investigate key Alzheimer’s disease regulators.
Syncell
How did researchers at the University of Stuttgart use Microscoop to study TNBC, and what did they learn?
Cristiana Lungu and her team at the University of Stuttgart used Microscoop to photolabel the nanometer-sized nuclear bodies of PRC2 in TNBC cells and then pulled them out of the sample for downstream analysis on a mass spectrometry instrument. This procedure was part of a broader study to identify novel regulatory mechanisms underlying the spatial organization of a protein that regulates TNBC development.
The unbiased nature of the Syncell Microscoop technology enabled the researchers to identify peptide enrichment candidates, including KDMA2 and PHF19. While these were known to affect chromatin regulation, PHF19 had not previously been studied for its molecular function in breast cancer cells. Lungu and her team found that PHF19 had a significant role in nuclear body formation and, thus, the metastatic phenotype in TNBC cells. Once PHF19 was knocked out, the nuclear bodies were dissolved and the motility of those cells decreased, indicating a reversal of the metastatic phenotype.
What is next on the horizon for Microscoop?
Scientists are just beginning to explore the impact of protein identification without predefined profiling or the use of probes and antibodies. We expect to see more use cases for the Microscoop as our customers employ the power of unbiased discovery in basic, translational, and drug discovery research. Microscoop can generate highly specific, sensitive, and extremely precise resolution, complementing the advancements in mass spectrometry like no other proteomic sample prep method.
As a company, Syncell is still young, and we’re building our commercial capabilities to support customers globally. In the last few months, we’ve hired commercial teams around the world and initiated a preferred provider network that can offer access to our technology as a service and to distribute our systems in China, Korea, and Japan. We plan on shipping to most regions in Europe and Asia by mid-year as the increased customer interest has accelerated our need to enable access to Microscoop. With all the new discoveries being made by our early adopters, we expect to start seeing some of their work in major conferences and pre-prints soon.
- Pelzer N, et al. PHF19 drives PRC2 sub-nuclear compartmentalization to promote motility in TNBC cells. bioRxiv. 2025;2025.03.13.642950.
- Anwar T, et al. p38-mediated phosphorylation at T367 induces EZH2 cytoplasmic localization to promote breast cancer metastasis. Nat Commun. 2018;9(1):2801.