Clinicians have used phages to treat bacterial infections since the early 20th century. Although the advent and mass production of antibiotics caused a decline in phage therapy, the recent rise in antibiotic resistance has renewed the global interest in this line of treatment. In this article, explore the discovery, types, applications, and challenges of phage therapy, along with strategies to overcome its limitations.
Phage therapy has the potential to treat patients who have severe infections caused by multidrug-resistant bacteria.
iStock
What Is Phage Therapy?
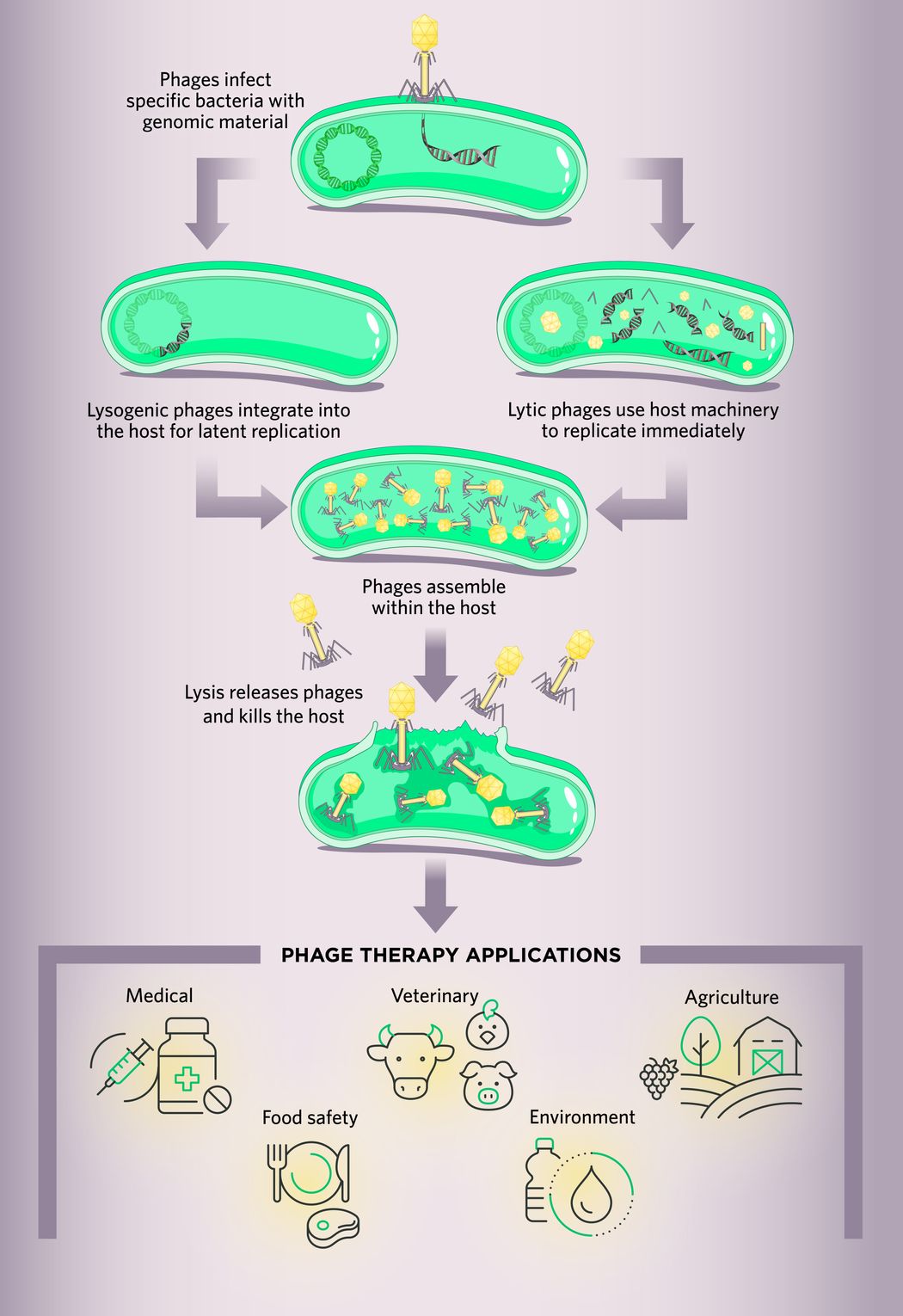
Phage therapy is a lifesaving intervention that uses viruses to treat bacterial infections.1 Phages, also known as bacteriophages, are viruses comprised of genomic material surrounded by a protein capsid. They infect specific bacteria and use host machinery to replicate via lytic or lysogenic cycles.2 For therapeutic purposes, scientists typically prefer lytic (virulent) phages over lysogenic (temperate) phages because the former has a more immediate impact, causing bacterial cells to burst and die.3
Lytic phages including T4 and T7 express proteins such as lysins that cause hydrolysis of a host cell’s peptidoglycan layer, leading to bacterial cell death.4 In contrast to lytic phages, lysogenic phages, such as lambda and P22, integrate their genetic material into the bacterial genome and rarely kill the host cells immediately.5 However, these phages may eventually cause cellular lysis.
Phage therapy helps combat the antibiotic resistance crisis by allowing scientists to target specific bacteria with bacteriophages that infect and destroy host cells without harming beneficial bacterial species.
Modified from © istock.com, ttsz, fonikum; designed by Erin Lemieux
Bacteriophage Infection
Each phage possesses specific features that influence bacterial infection. For clinical applications, researchers mostly use bacteriophages belonging to the order Caudovirales, also known as tailed phages. These phages contain receptor binding proteins in their tail fibers, which recognize and bind to specific host receptors present on bacterial cell walls, appendages (e.g., pili and flagella), or polysaccharide capsules.
After binding the bacterial receptor, the phage undergoes a conformational change through complex mechanisms that ultimately open a channel between the phage and host, allowing the phage’s genomic materials to enter the bacterial cell. Thereafter, the phage utilizes the host's machinery to synthesize multiple copies of itself. If the infecting phage is virulent, the infection follows a lytic cycle, or for temperate phages, a lysogenic cycle occurs.
Rise, Fall, and Resurgence of Phage Therapy
Microbiologist Félix Hubert d'Herelle first reported bacteriophages in 1917 and described them as obligate intracellular parasites of bacteria.6 He proposed the use of phages as prophylactic and therapeutic agents against various bacterial infections because of their unique antibacterial properties.7
Because of d’Herelle’s research, clinicians in the US began using bacteriophages therapeutically around 1922; however, the subsequent discovery of antibiotics, their quick production, long shelf-life, high efficacy against wide ranging pathogens, and widespread usage during World War II led to a decline in phage therapy.8 Compared to antibiotics, early phage therapies were difficult to purify and store, and yielded inconsistent efficacy.9
Over the years, antibiotics have been misused worldwide, including by physicians prescribing them for viral infections and through their excessive use in animal husbandry and agriculture for disease prevention and enhanced plant growth. This overuse or misuse had led to a rise in antibiotic resistance, which currently poses a major threat to global health. This increase revived phage therapy in the early 2000s.10
Phage Therapy Approaches
Scientists have developed non-personalized and personalized phage therapy approaches.10 Non-personalized phage therapy is a one-size-fits-all pre-defined formulation that consists of several bacteriophage strains, while personalized phage therapy comprises active phages that are specifically selected from a pre-existing phage library according to a specific patient’s need.
In comparison to broad-spectrum antibiotics, viruses used for personalized phage therapy infect only targeted pathogenic bacteria without harming beneficial species, which reduces the risk of phage resistence.10
Depending on the mode of therapeutic action, scientists have categorized phage therapy into five types: conventional therapy, modified phage therapy, therapy with enzymes derived from phages, therapy with proteins derived from phages, and combination therapy.12 Clinical trials have revealed that personalized phage therapy in combination with antibiotics exhibits superior clinical improvement in patients with a bacterial infection.11
Table 1: Different phage therapy types for bacterial infection treatment12
Therapy | Phage types | Mode of actions | Target pathogens
|
Conventional phage therapy(e.g., monophage therapy and pyophage therapy)
| Naturally occurring virulent phages |
|
|
Modified phage therapy | Genetically engineered lysis-deficient phages |
|
|
Therapy with enzymes derived from phages | Phage enzymes specific for Gram positive bacteria (e.g., endolysins) |
|
|
Therapy with proteins derived from phages | Phage proteins specific for Gram negative bacteria (e.g., virion-associated peptidoglycan hydrolases (VAPGHs) and enzyme depolymerases) |
|
|
Combination therapy | Antibiotics (e.g., chloramphenicol) can be linked to the phage protein coat by an aminoglycoside bond |
|
|
Phage Therapy Applications
Phage therapy is a promising candidate for combating the antibiotic resistance crisis because of its capacity to target and infect specific bacteria. Another advantage of using phages to alleviate bacterial infection is their ability to self-amplify, which potentially reduces the need for repeated dosing.13
Besides tackling antibacterial resistance, scientists use phages to combat other problems including the following.
- Medical: Phage therapy can treat chronic diseases where bacteria contribute to pathogenesis. For example, scientists have identified a prophage active against Helicobacter pyroli, indicating the possibility of a new phage therapy strategy that could target the etiological role of this pathogen in gastric cancer and gastric ulcers.14 Clinicians also use phages as prophylaxis to prevent infection among individuals who were in close contact with patients infected with highly transmissible bacterial pathogens such as Vibrio cholerae.15
- Veterinary: At the beginning of the 21st century, phages were orally introduced to reduce Salmonella colonization in broiler chickens.16 Phage therapy can effectively alleviate mastitis in bovines caused by several bacterial species including those from the Streptococcus and Staphylococcus genera.17
- Agriculture: Phage cocktail treatment prevents Pierce’s disease in grapevines, caused by Xylella fastidiosa subsp. fastidiosa.18
- Food safety: Scientists are currently assessing the efficacy of phages as food product decontaminants, for example, to decontaminate meat from Campylobacter jejuni.19
- Environment: Researchers are investigating applications for bacteriophages as biocontrol agents in wastewater treatment.20
Challenges and Future Considerations
Although phage treatment exhibits significant benefits, it is not easy to formulate phage cocktails with broad-ranging efficacy similar to antibiotics. Bacteriophages are highly specific and act only on certain bacterial genera. Sometimes they even fail to target all pathogenic strains within a bacterial species.21 In most clinical cases, patients may contract a variety of pathogenic bacteria, which may limit the desired therapeutic effect of phage treatment.
Personalized phage therapy may require specific phages that are not universally accessible across phage therapy centers. Therefore, scientists often face difficulty in formulating personalized phage therapy because of logistic limitations, and patients may need to be transferred to specific centers for treatment. To overcome logistical challenges, scientists have begun developing point-of-care phage production opportunities using synthetic genomic methodologies to create phage-sized genomes in a permissive bacterial host or via cell-free transcription-translation (TXTL) systems.22
There are no standard protocols for phage isolation, purification, or clinical use, which leads to variable therapeutic efficacy.23 The absence of standard protocols for developing phage cocktails also hinders scientists from comparing different phage treatments and their outcomes.
Various strategies, including the creation of Phagistry, an international registry that systematically collects data related to diagnosis, phages, treatments, and outcomes for patients receiving phage therapy, have significantly contributed to enhanced phage therapy development. Furthermore, the effective collaboration of multiple stakeholders including researchers, clinicians, investors, and regulatory bodies will increase phage therapy's applicability.22
- Sawa T, et al. Current status of bacteriophage therapy for severe bacterial infections. J Intensive Care. 2024;12(44):1-9.
- Erez Z, et al. Communication between viruses guides lysis–lysogeny decisions. Nature. 2017;541(7638):488-493.
- Venturini C, et al. Biological foundations of successful bacteriophage therapy. EMBO Mol Med. 2022;14(7):e12435.
- Ioannou P, et al. Bacteriophages in infectious diseases and beyond-A narrative review. Antibiotics (Basel). 2023;12(6):1012.
- Gummalla VS, et al. The role of temperate phages in bacterial pathogenicity. Microorganisms. 2023;11(3):541.
- Summers WC. Félix Hubert d'Herelle (1873-1949): History of a scientific mind. Bacteriophage. 2017;6(4):e1270090.
- Summers WC. The strange history of phage therapy. Bacteriophage. 2012;2(2):130-133.
- Aswani VH, Shukla SK. An early history of phage therapy in the United States: Is it time to reconsider? Clin Med Res. 2021;19(2):82-89.
- Sahoo K, Meshram S. The evolution of phage therapy: A comprehensive review of current applications and future innovations. Cureus. 2024;16(9):e70414.
- Pirnay J. Phage therapy in the year 2035. Front Microbiol. 2020;11:538375.
- Diallo K, Dublanchet A. Benefits of combined phage-antibiotic therapy for the control of antibiotic-resistant bacteria: A literature review. Antibiotics (Basel). 2022;11(7):839.
- Kapoor A, et al. Phage therapy: A novel approach against multidrug-resistant pathogens. 3 Biotech. 2024;14:256.
- Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1(2):111-114.
- Ferreira R, et al. Characterization and genomic analysis of a new phage infecting Helicobacter pylori. Int J Mol Sci. 2022;23(14):7885.
- Bhandare S, et al. Reviving phage therapy for the treatment of cholera. J Infect Dis. 2019;219(5):786-794.
- Żbikowska K, et al. The use of bacteriophages in the poultry industry. Animals (Basel). 2020;10(5):872
- Nale JY, McEwan NR. Bacteriophage therapy to control bovine mastitis: A review. Antibiotics (Basel). 2023;12(8):1307.
- Das M, et al. Control of Pierce's disease by phage. PLoS One. 2015;10(6):e0128902.
- Olson EG, et al. Application of bacteriophages to limit Campylobacter in poultry production. Front Microbiol. 2022;12:458721.
- Runa V, et al. Bacteriophages in biological wastewater treatment systems: Occurrence, characterization, and function. Front Microbiol. 2021;12:730071.
- Hyman P, Abedon ST. Bacteriophage host range and bacterial resistance. Adv Appl Microbiol. 2010;70:217-48.
- Strathdee SA, et al. Phage therapy: From biological mechanisms to future directions. Cell. 2023;186(1):17-31.
- Lin J, et al. Limitations of phage therapy and corresponding optimization strategies: A review. Molecules. 2022;27(6):1857.
- Yang Q, et al. Regulations of phage therapy across the world. Front Microbiol. 2023;14:1250848.
















