Humans have been attempting to combat cancer since the time of the Ancient Egyptians, and recent decades have seen remarkable progress. However, tumor cells have developed sophisticated ways to evade detection, including hijacking the immune system’s brake mechanism, known as immune checkpoints. In this article, explore how the research into blocking these checkpoints has led to immune checkpoint inhibitors (ICIs), a promising but still evolving class of immunotherapy that falls under the broader category of biologics.

Immune checkpoint inhibitors help boost natural immune activity against tumor cells. This field of immunotherapy has seen remarkable growth over the past two decades.
iStock, SeventyFour
Introduction to Checkpoint Inhibitors
In 1891 at New York Hospital, after reading reports of infection and high fever triggering tumor regression, clinician scientist William Bradley Coley began injecting patients’ tumors with live and inactivated bacterial strains.1 Over time, he documented tumor regression in dozens of patients treated with inactivated bacteria, later known as Coley’s toxins. Although Coley’s toxins are not used in cancer treatment today, he is recognized for helping establish the field of immunotherapy. Still, significant clinical breakthroughs in cancer immunotherapy took decades to emerge.
In 2018, Tasuku Honjo and James Allison won the Nobel Prize for their respective work on the immune checkpoint proteins programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4).2 In 1992, Honjo identified PD-1, an immune checkpoint protein expressed by T cells that functions as a negative regulator of the immune response.3 Over the next decade, Honjo and his research team meticulously investigated its mechanism, ultimately discovering that blocking PD-1 could restore T cells' ability to combat cancer in animal models.3 In 1994-1995, Allison and his colleagues began focusing on CTLA-4, a protein discovered in the 1980s and known to function as a brake on the immune system. Allison was the first to demonstrate that using an antibody to block CTLA-4 could effectively release this inhibitory signal and restore the immune system’s ability to target tumors.4
The pioneering work of Allison and Honjo laid the foundation for an expanding field of cancer therapeutics and ICIs. Research on CTLA-4, PD-1, and other immune checkpoints continues to show that blocking these so-called brakes can enhance the immune system's ability to fight cancer. These discoveries continue to reshape cancer treatment, paving the way for immunotherapies that have significantly improved patient outcomes worldwide, though further research and optimization are needed to maximize their effectiveness.5
How Do Immune Checkpoint Inhibitors Work?
To understand how ICIs work, it is essential to first grasp how the immune system activates, how immune checkpoints function, and how inhibiting these checkpoints can enhance antitumor activity.
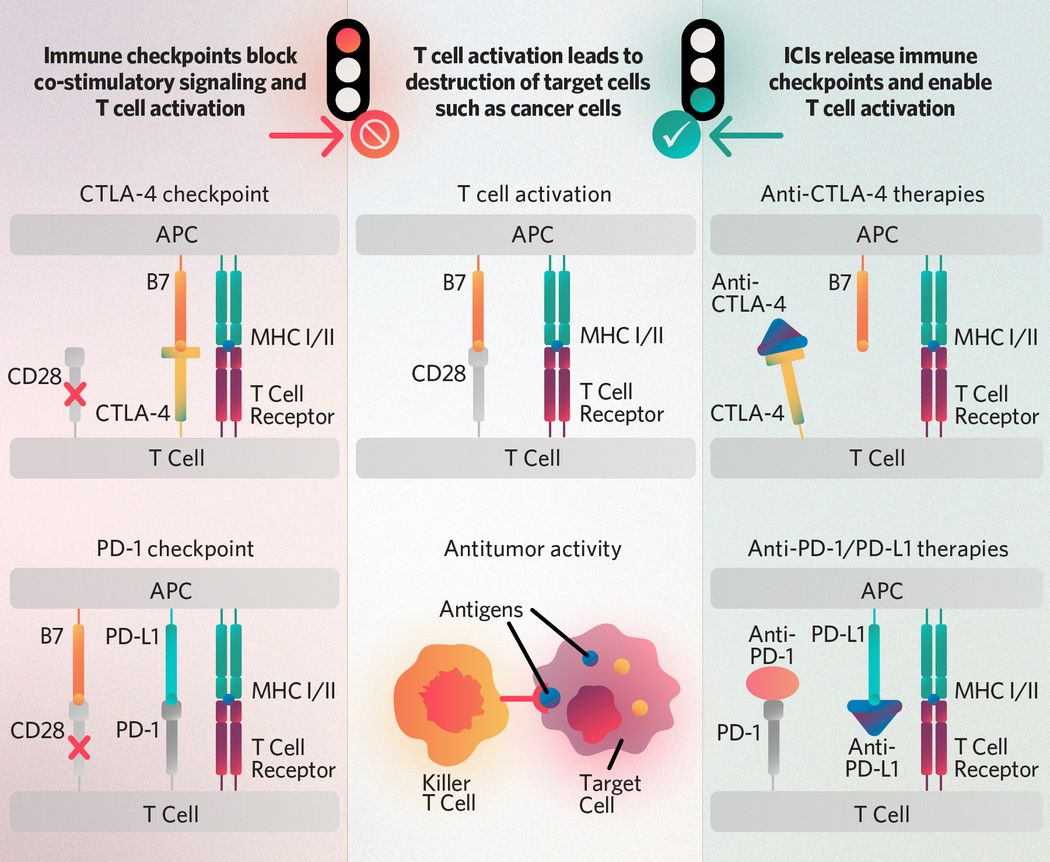
T cell receptors recognize antigens presented on the major histocompatibility complex (MHC I/II) of antigen-presenting cells (APCs) and receive activation signals from co-stimulatory molecule interactions such as those between CD28 and APC-expressed B7-family ligands. The CTLA-4 and PD-1 checkpoints block co-stimulatory activation signaling, and immune checkpoint inhibitors (ICIs) such as anti-CTLA-4, anti-PD-1, and anti-PD-L1 therapies enable it. Once activated, T cells can recognize antigens presented by cancer cells and target tumors for destruction.
The Scientist
T cell activation
The human immune system consists of various specialized cells that work together to fight infections and cancer. Among the most crucial are T lymphocytes, or T cells. There are several classes of T cells including CD4+ helper T cells, CD8+ cytotoxic T cells, regulatory T cells, and memory T cells. For CD4+ and CD8+ T cells to function properly, they require two activation signals.5 First, they must recognize an antigen presented on the major histocompatibility complex (MHC) of antigen-presenting cells (APCs).5 Second, CD4+ and CD8+ T cells receive a signal from co-stimulatory molecules for full activation. For example, CD28, a key co-stimulatory protein on T cells, binds to its counterparts B7-1 (CD80) and B7-2 (CD86) expressed by APCs. This interaction triggers a signal transduction cascade that fully activates the T cell and ensures an optimal immune response.5
Immune checkpoints
Just as the human body has mechanisms to activate T cells, it also has inhibition mechanisms that help regulate the immune system and prevent excessive activity such as autoimmune reactions. This occurs in part through co-inhibitors, or immune checkpoints, which serve in contrast to co-stimulators. The most common immune checkpoints include the following.
- CTLA-4 is a checkpoint molecule expressed by recently activated T cells. It prevents T cell activation by interfering with the CD28:B7 interaction.6 This inhibitory effect is particularly useful when B7 levels are low, allowing CTLA-4 to outcompete the lower-affinity CD28 receptor.6 CTLA-4 also inhibits the immune response by removing B7 molecules from APCs and by enhancing the activity of immunosuppressive regulatory T cells.7 CTLA-4 signaling can also halt intrinsic T cell transcription factors involved activation.6
- PD-1 is an inhibitory receptor of the CD28 family. It binds to the APC-expressed ligands PD-L1 (B7-H1) and PD-L2 (B7-DC). Upon ligand binding, PD-1 recruits tyrosine phosphatases that inhibit signal transduction between the T cell receptor and its co-stimulatory molecule, CD28, thereby suppressing activated T cells and dampening the immune response.6
Immune checkpoint inhibitors
Due to the inhibitory effects of CTLA-4, PD-1, and PD-1 ligands on the immune response, researchers early on proposed that blocking these pathways may enhance lymphocyte activation and antitumor activity.
This was supported by the fact that tumor cells exploit immune checkpoint mechanisms to evade immune detection, upregulating immune checkpoint proteins in the tumor microenvironment (TME).8 For example, in various cancers, including gastric, hepatocellular, renal cell, pancreatic, and ovarian cancers, tumor cells overexpress PD-L1.9 Research indicates that host cells within the TME and lymph nodes—such as APCs, fibroblasts, and T cells—also upregulate PD-L1 expression. Similarly, certain solid and hematologic malignancies exhibit CTLA-4 expression.10 Scientists found that blocking these checkpoint molecules with antibodies strengthens the immune response to tumors, establishing a basis for the therapeutic approach known as checkpoint blockade. This discovery has fueled extensive research into ICIs as cancer treatments over the past two decades.
In 2011, a CTLA-4 inhibitor called ipilimumab, became the first FDA-approved ICI.11 Initially used to treat metastatic melanoma, ipilimumab is now approved for various other malignancies. Since its approval, scientists have introduced several other ICIs targeting CTLA-4, PD-1, and PD-L1, with more currently in development.11
Emerging Inhibitors
While CTLA-4, PD-1, and PD-L1 are the primary focus of current ICIs, there are several other immune regulatory molecules that scientists are investigating as potential future targets for ICI therapies.
- Lymphocyte activation gene-3 (LAG-3): Also known as CD223, LAG-3 is an inhibitory receptor and a promising immunotherapeutic target.12 In fact, it is already the basis of an FDA-approved melanoma treatment. While its precise mechanism of action remains under study, LAG-3 competitively binds to MHC-II. This prevents the interaction between MHC-II and the T cell receptor (TCR)–CD3 complex, thereby inhibiting T cell proliferation and effector T cell activation. Additionally, LAG-3 enhances immunosuppressive regulatory T cell function. In 2022, the FDA approved a LAG-3 checkpoint inhibitor for use alongside PD-1/PD-L1 inhibitors in patients with unresectable metastatic melanoma.13
- T cell immunoglobulin and mucin domain-3 (TIM-3): This surface glycoprotein is highly expressed on exhausted and dysfunctional T cells.14 Unlike other inhibitory molecules, TIM-3 lacks a conventional inhibitory motif. Instead, it operates through a unique mechanism, toggling between activation and inhibition. When unbound, TIM-3 promotes normal T cell responses and antitumor activity. Upon binding to its ligands in the tumor microenvironment, it undergoes phosphorylation, conformational changes, and co-factor recruitment, eventually leading to the inhibition of T cell function and even cell death. Current research indicates that co-targeting of the TIM-3 and PD-1 pathways has efficacy in combating solid tumors.
- T cell immunoglobulin and ITIM domain (TIGIT): This immune checkpoint binds to its ligands, CD115 and CD112, which are both members of the poliovirus receptor (PVR) family.15 This binding suppresses TCR expression and T cell signaling. Additionally, TIGIT is a competitive inhibitor of CD226, a co-stimulatory receptor that activates T cells in response to PVR. TIGIT outcompetes CD226 for binding to PVR. This checkpoint molecule is an emerging focus in ICI research. Ongoing studies and clinical trials are evaluating TIGIT’s efficacy in treating cancers, both as a monotherapy and in combination with PD-1/PD-L1 inhibitors.16
- V-domain Ig suppressor of T cell activation (VISTA): This immune checkpoint is commonly expressed on naïve T cells, inhibiting T cell activity early in the immune response.17 Researchers are still investigating VISTA’s role in tumor immune surveillance, but early clinical trials evaluating its efficacy as a form of immunotherapy are underway.18
Response to Treatment and Current Outlook
Oncologists tailor cancer treatments to individual cases—a therapy that works for one cancer type may not work for another. Immune checkpoint inhibitors are no exception, as responses can vary widely. To address this, scientists are identifying biomarkers that can predict optimal responses to ICIs. Currently, a few FDA-approved predictive biomarkers exist for the immune response to immune checkpoint inhibitors.19
- PD-L1 protein expression: PD-L1 testing has been approved for several tumor types. Scientists use various methods to assess PD-L1 expression, such as immunohistochemistry and antibody testing, and each method has its own scoring system and threshold cutoffs for efficacy.20 This is a notable limitation that can cause confusion for researchers, clinicians, and patients. Furthermore, despite being the most common predictive biomarker, PD-L1’s accuracy in assessing the efficacy of ICIs is generally poor. Notably, some studies have shown that up to 20 percent of patients with PD-L1-negative tumors respond to treatment with PD-1/PD-L1 antibodies.19
- Microsatellite instability (MSI)/DNA mismatch repair (dMMR): The dMMR system is essential for recognizing and repairing base errors that occur during DNA replication. Tumors with suboptimal dMMR develop thousands of mutations throughout their genetic code, particularly in microsatellite regions—short stretches of DNA roughly one to six nucleotides long. When errors accumulate in these regions, it is known as microsatellite instability (MSI). In 2017, MSI/dMMR became the second FDA-approved predictive biomarker for ICI response. Testing options include immunohistochemistry to assess the loss of four key dMMR proteins—MLH1, MSH2, MSH6, and PMS2—as well as polymerase chain reaction (PCR) or next-generation sequencing (NGS) testing.19
- Tumor mutational burden: This is the third FDA-approved biomarker for ICIs. Using sequencing techniques, researchers quantify the number of mutations in cancer cell DNA, reported as mutations per megabase (mut/Mb).21 Genomic damage caused by smoking, ultraviolet radiation, and dMMR all contribute to high TMB. Research on TMB cutoffs for predicting ICI response is still ongoing and a subject of debate.19
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154-158.
- Huang PW, Chang JWC. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed J. 2019;42(5):299-306.
- Ishida Y. PD-1: its discovery, involvement in cancer immunotherapy, and beyond. Cells. 2020;9(6):1376.
- Leach DR, et al. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-1736.
- Sharma P, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186(8):1652-1669.
- Wei SC, et al. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069-1086.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
- Toor SM, et al. Immune checkpoints in the tumor microenvironment. Semin Cancer Biol. 2020;65:1-12.
- Cha JH, et al. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76(3):359-370.
- Oyewole-Said D, et al. Beyond T-Cells: Functional characterization of CTLA-4 expression in immune and non-immune cell types. Front Immunol. 2020;11:608024.
- Lee JB, et al. Immune checkpoint inhibitors in 10 years: Contribution of basic research and clinical application in cancer immunotherapy. Immune Netw. 2022;22(1):e2.
- Long L, et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9(5-6):176-189.
- Kreidieh FY, Tawbi HA. The introduction of LAG-3 checkpoint blockade in melanoma: immunotherapy landscape beyond PD-1 and CTLA-4 inhibition. Ther Adv Med Oncol. 2023;15:17588359231186027.
- Acharya N, et al. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8(1):e000911.
- Cai L, et al. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16(1):101.
- Zhang P, et al. Targeting TIGIT for cancer immunotherapy: recent advances and future directions. Biomark Res. 2024;12(1):7.
- Huang X, et al. VISTA: An immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol. 2020;13(1):83.
- Zhang RJ, Kim TK. VISTA-mediated immune evasion in cancer. Exp Mol Med. 2024;56(11):2348-2356.
- Wang Y, et al. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front Oncol. 2021;11:683419.
- Doroshow DB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345-362.
- Klempner SJ, et al. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: A review of current evidence.The Oncologist. 2020;25(1):e147-e159.














